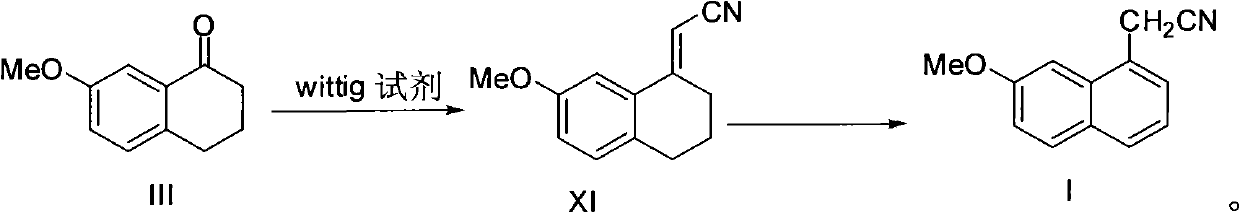
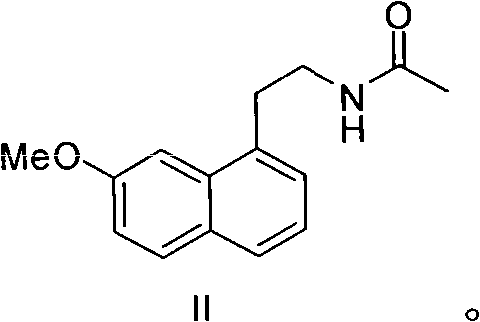
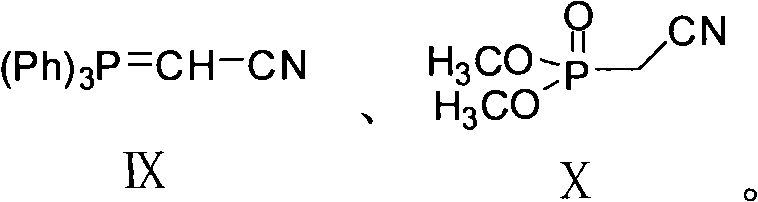Preparation method of 2-(7-methoxy-1-naphthyl) acetonitrile
A methoxyl and naphthyl technology, applied in a new preparation field, can solve the problems of harsh reaction requirements, environmental pollution, incomplete reaction, etc., and achieve the effects of mild reaction conditions, high product purity, and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Dissolve 35.2 g of compound III (7-methoxytetralone) and 60.2 g of compound IX in 340 ml of toluene, heat to 100° C. until the raw materials disappear, and cool to room temperature. Wash and dry. The solvent was distilled off to obtain 36.0 g of compound XI. Yield: 90%. ESI-MS: [M+H] + 200.
Embodiment 2
[0032]
[0033] Dissolve 35.2 g of compound III (7-methoxytetralone) and 30.0 g of compound X in 170 ml of toluene, carefully add 13.6 g of sodium ethoxide, heat to 100°C until the raw materials disappear, and cool to room temperature. Wash and dry. The solvent was distilled off to obtain 36.5 g of compound XI. Yield: 91%. ESI-MS: [M+H] + 200.
Embodiment 3
[0035]
[0036] 36.0 g of compound XI prepared in Example 1 and 7.2 g of 10% Pd / C were dissolved in 180 ml of toluene, heated to reflux until the raw materials disappeared, and cooled to room temperature. Suction filtration, washing and drying. The solvent was distilled off to obtain the target compound I (30.6 g). Yield: 85%. Melting point: 83-84°C. ESI-MS: [M+H] + 198. 1 H-NMR (CDCl 3 , ppm): 7.20-7.79 (m, 5H, ArH), 7.0 (d, 1H, J=2.1Hz, ArH), 4.1 (s, 2H, ArCH 2 ), 3.94 (3, 3H, OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com