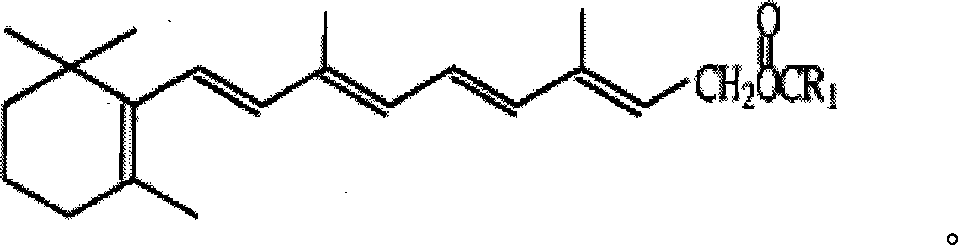
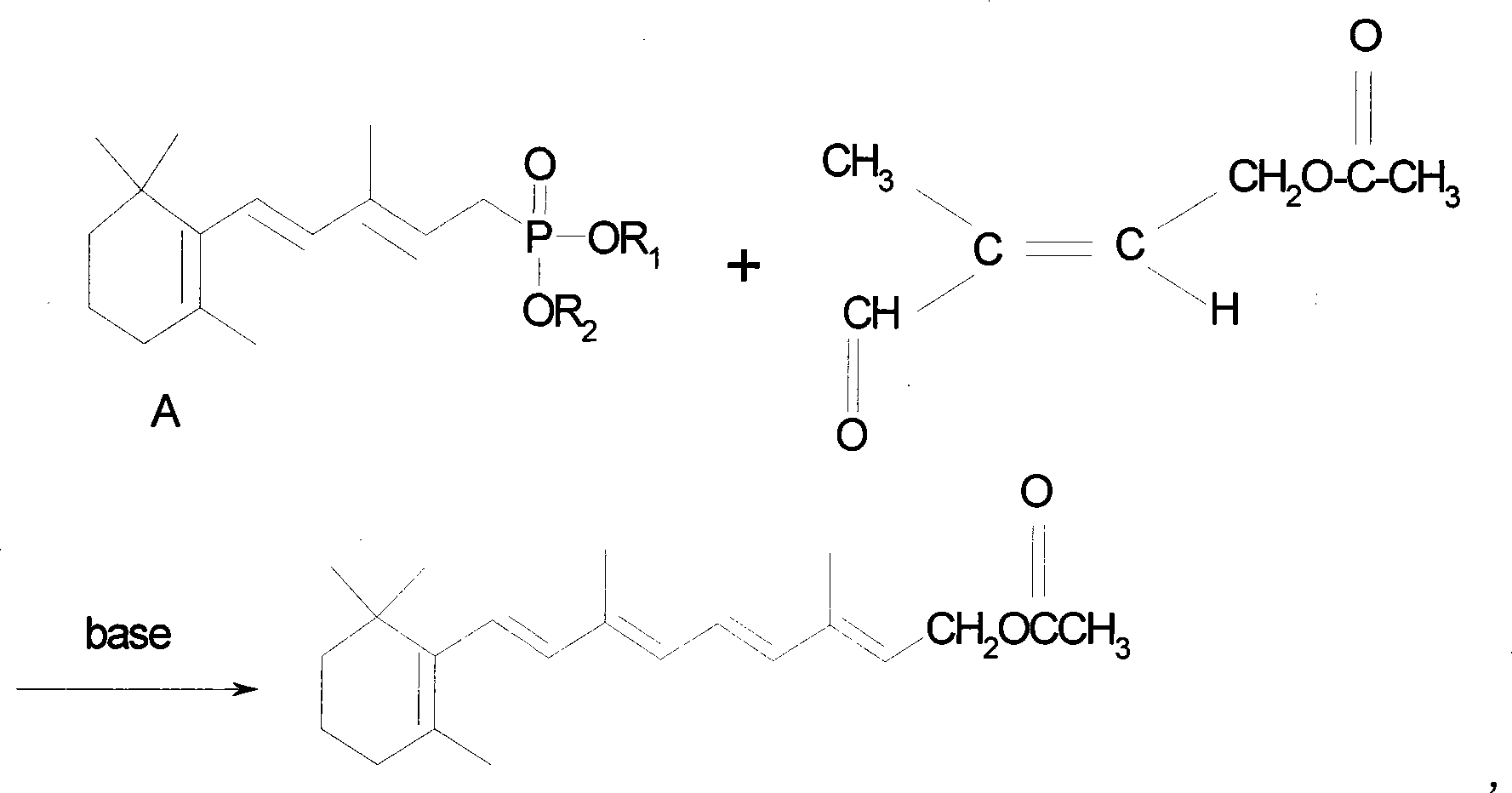
Method for producing improved vitamin A acetic ester
A technology of acetate and vitamin, which is applied in the field of preparation of vitamin A acetate, can solve the problems of high difficulty in industrial operation, high energy consumption, unfavorable large-scale production, etc., and achieve the reduction of energy consumption and the difficulty of production operation, high Yield, the effect that is conducive to industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Diethyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienylphosphonate (10g, content 95.5% , 27.5mmol) was dissolved in 80ml of toluene, then 1.5g of sodium methoxide and 20ml of pyridine were added, the temperature was adjusted and maintained at 20°C, and stirred under nitrogen protection for 5 minutes, the 4-acetoxy-2-methyl- 2-butene-1 aldehyde (5g, content is 99%, 35mmol) is added dropwise in the toluene solution of 20ml with the time of 20 minutes, continues to react for half an hour; Add water 150ml, stir layering, separate and take organic layer, Dry and evaporate to dryness under reduced pressure to obtain 11.5g of yellow solid; add 100ml of methanol, cool to -10°C, stand for 3 hours, crystallize, filter to obtain 10.5g of light yellow solid, according to the appendix of "Chinese Pharmacopoeia 2005 Edition" (Part II) The first method in VIIJ vitamin A determination method (Appendix 45-47 pages), the detection yield is 91.5%.
example 2
[0024] Diethyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadienylphosphonate (10g, content 95.5% , 27.5mmol) was dissolved in 80ml of toluene, then 2g of sodium ethoxide and 25ml of 3-picoline were added, the temperature was maintained at 30°C, and stirred under nitrogen protection for 5 minutes, the 4-acetoxy-2-methyl- 2-butene-1 aldehyde (5g, content is 99%, 35mmol) is added dropwise in 20ml of toluene solution with the time of 20 minutes, continue to react for half an hour after dropping, add water 150ml, stir layering, separate The organic layer was taken, dried, and evaporated to dryness under reduced pressure to obtain 11.5 g of a yellow solid, then 100 ml of methanol was added, cooled to -10° C., left for 3 hours, crystallized, and filtered to obtain 9.5 g of a light yellow solid. According to "Chinese Pharmacopoeia 2005 Edition" ( Part 2) The first method in the vitamin A determination method of Appendix VIIJ (appendix 45-47 pages), the detection yield is 8...
example 3
[0026] Dipropyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,4-pentadienylphosphonate (10g, content 95.5% , 25.2mmol) was dissolved in 85ml of toluene, then 1.8g of sodium ethoxide and 20ml of 2,5-lutidine were added, the temperature was maintained at 25°C, and the nitrogen protection was stirred for 10 minutes, and the 4-acetoxy-2 -Methyl-2-butene-1 aldehyde (5g, content is 99%, 35mmol) is added dropwise in the toluene solution of 10ml with the time of 15 minutes, continue to react for 20 minutes after dropping, add water 150ml, stir to divide layer, the organic layer was separated, dried, evaporated to dryness under reduced pressure to obtain 11g of yellow solid, then added 100ml of methanol, cooled to -10°C, left for 3 hours, crystallized, filtered to obtain 10g of light yellow solid, according to "Chinese Pharmacopoeia 2005 Edition" (Part Two) The first method in the vitamin A assay method of Appendix VIIJ (appendix 45-47 pages), the detection yield is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com