Method for preparing dimethyl furan-2,5-dicarboxylate by oxidizing and esterifying 5-hydroxymethyl furfural
A technology of dimethyl diformate and hydroxymethylfurfural, which is applied in organic chemistry and other fields, can solve the problems of low yield and harsh reaction conditions, and achieve the effects of high selectivity, short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
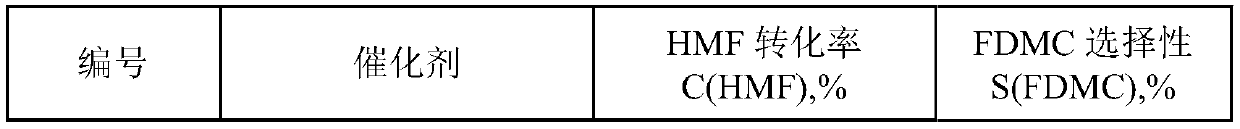
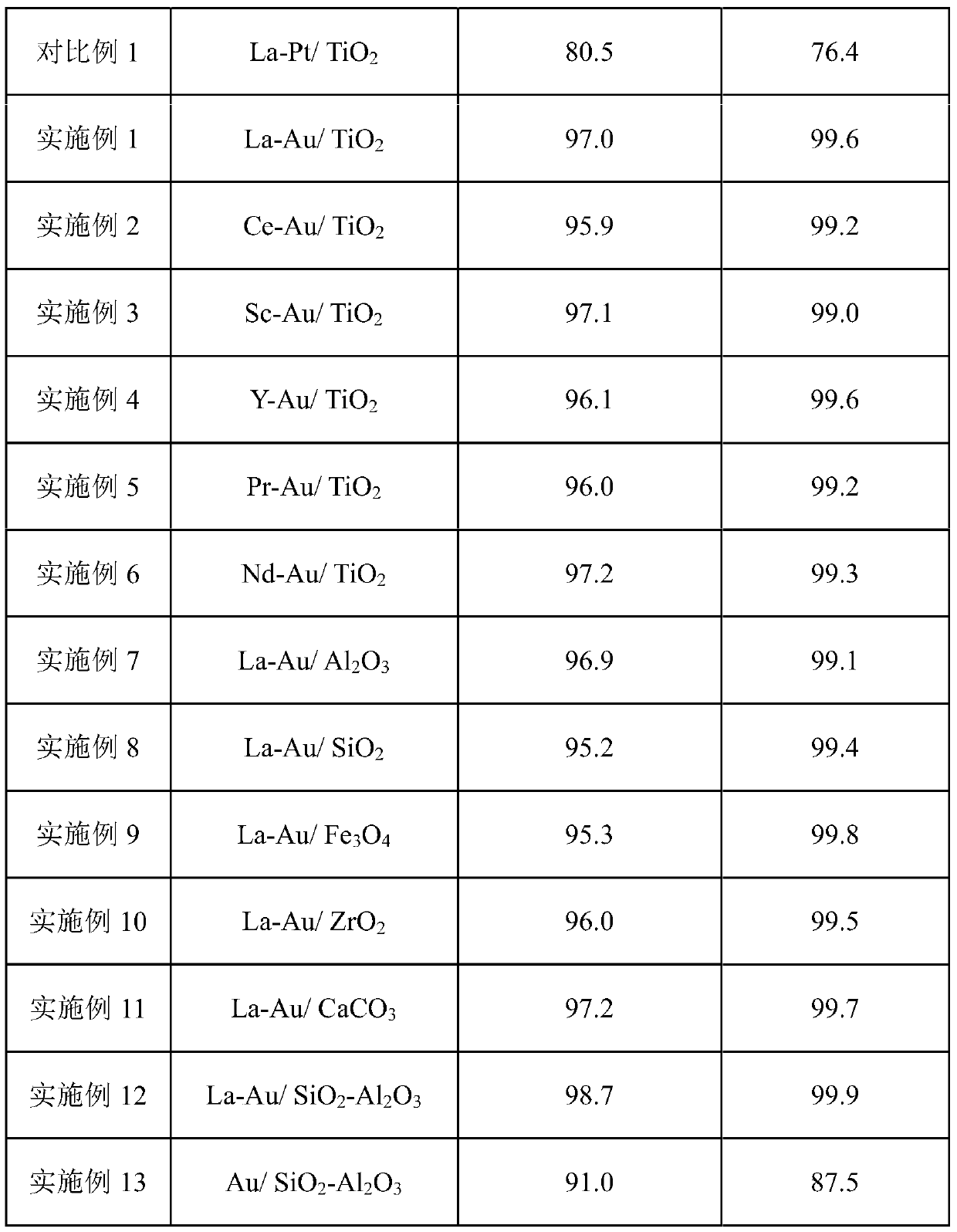
[0039] Under room temperature conditions, 1.6g of chloroauric acid and 1g of sodium citrate were dissolved in 600mL of deionized water during stirring, and after fully dissolving, 1g of polyvinylpyrrolidone (PVP, molecular weight 8000-10000) and 9.4g of lanthanum nitrate were added, completely Add 0.3kg TiO after dissolving2 Powder, continue to stir and slowly raise the temperature to 75°C, continue to stir at this temperature for 14 hours and then cool down to room temperature, pour out the upper liquid after standing still, wash the lower precipitate with deionized water until no chloride ions are detected in the solution, and dry at 100°C After 24 hours, it was calcined in air at 300°C for 24 hours to obtain the catalyst La-Au / TiO 2 . The mass percentages of La and Au in the catalyst are 1% and 0.1% respectively.
Embodiment 2
[0041] Catalyst preparation condition is the same as embodiment 1, and lanthanum nitrate is replaced with cerium nitrate, obtains catalyst Ce-Au / TiO 2 , wherein the mass percentages of Ce and Au in the catalyst are 1% and 0.1% respectively.
Embodiment 3
[0043] Catalyst preparation condition is the same as embodiment 1, and lanthanum nitrate is replaced with cerium nitrate, obtains catalyst Sc-Au / TiO 2 , wherein the mass percentages of Sc and Au in the catalyst are 1% and 0.1% respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com