Furanosyl modified 1,3,4-thiadiazole derivative and preparation method thereof as well as application of derivative as bactericide
A bactericide, phenyl technology, applied to furanosyl-modified 1,3,4-thiadiazole derivatives and their preparation and application as a bactericide, can solve the complex synthesis of glycosyl isothiocyanates , high preparation cost, difficult separation and purification, etc., to achieve the effects of good growth inhibition, cheap raw materials, and simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
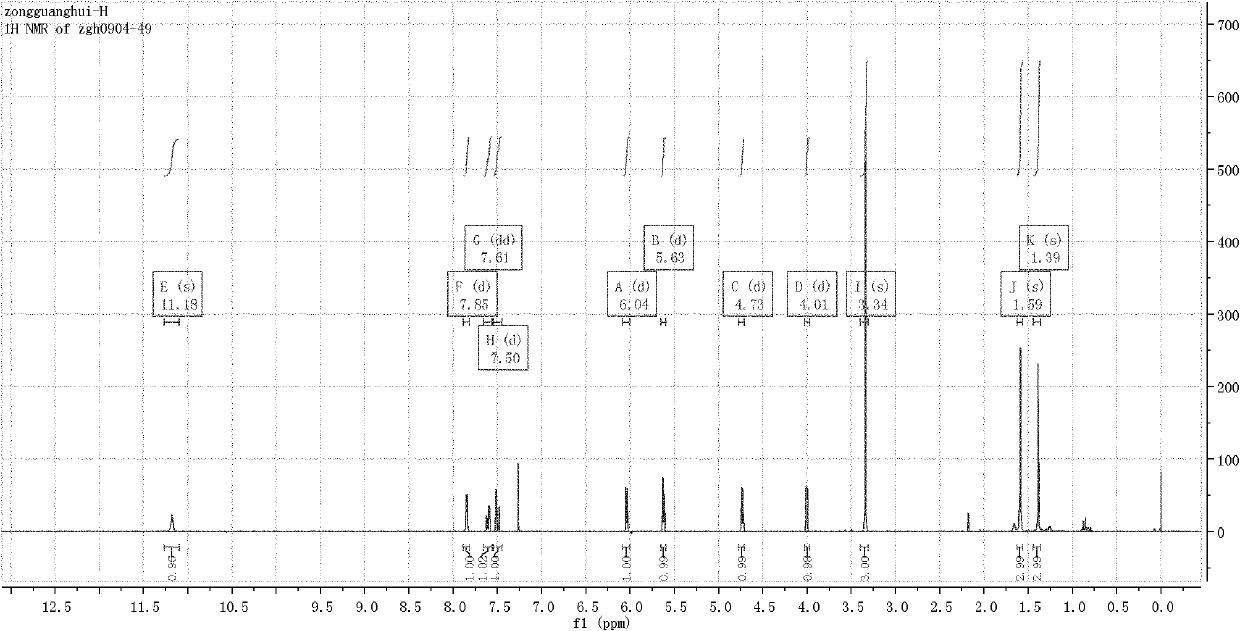
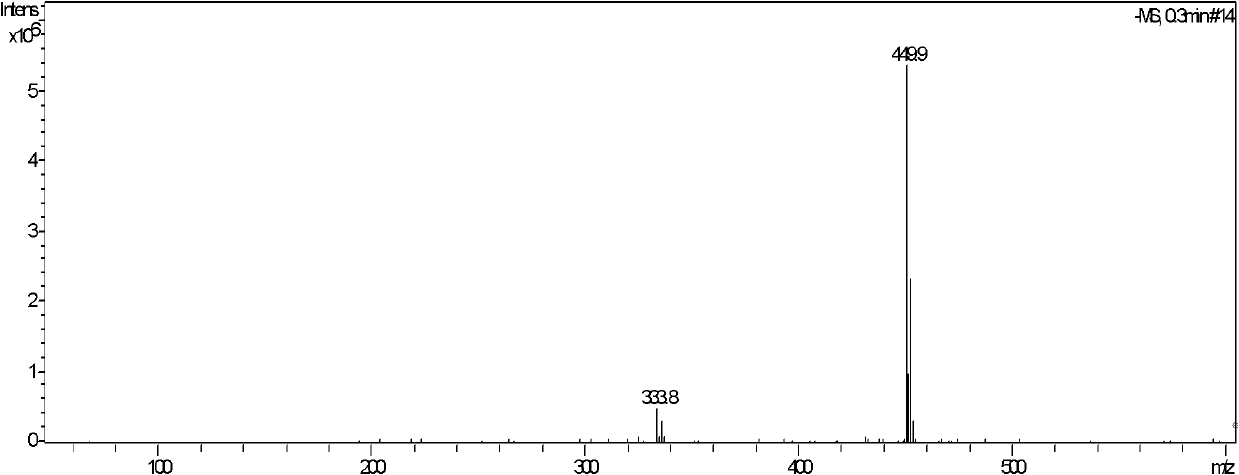
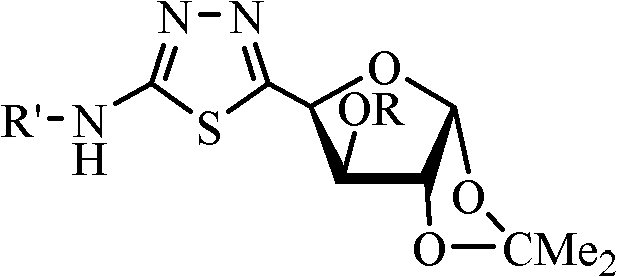
[0042] Example 1. Compound CAU-2010-ZJ-A-1 (R=Ac (acetyl) in formula I, R'=4-Cl-2-CF 3 -Phenyl) preparation and structure identification.
[0043]
[0044] In a 100mL round-bottom flask, add 1,2-O-isopropylidene-3-O-acetyl-xylofuranose-5-aldehyde 1.15 g (2, R is acetyl), N-4-Cl-2 -CF 3-Phenylthiosemicarbazide (1.48g, 5.5mmol), dissolved in 30mL of anhydrous dichloromethane, heated to reflux for 6h, directly concentrated after the reaction, the concentrate can be directly entered into the next step reaction, or the concentrate can be quickly Preliminarily separated through a short silica gel column or recrystallized with petroleum ether / ethyl acetate (v / v=6:1) to precipitate a white powder, and the obtained solid was dried and then proceeded to the next step. The obtained crude product was dissolved in an appropriate amount of chloroform, 3.8 g of manganese dioxide was added, and stirred at room temperature for 2 h. After the reaction, the manganese dioxide was removed by ...
Embodiment 2
[0048] Embodiment 2, the preparation of compound CAU-2010-ZJ-A-1 emulsifiable concentrate
[0049] Add compound CAU-2010-ZJ-A-11~10g, emulsifier 5~15g, penetrant 0.1~1g into a 100ml volumetric flask, and then use solvent such as toluene, xylene, etc. to make up the content of 1~10% cream.
[0050] Other emulsifiable concentrates with the general formula of CAU-2010-ZJ compound can be prepared according to the above method.
Embodiment 3
[0051] Embodiment 3, the preparation of compound CAU-2010-ZJ-A-1 wettable powder
[0052] Take compound CAU-2010-ZJ-A-115-50g, surfactant 10-20g, white carbon black 30-75g, mix and pulverize to obtain a wettable powder with a content of 15-50%.
[0053] Wettable powders of other compounds with general formula CAU-2010-ZJ can be prepared according to the above method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com