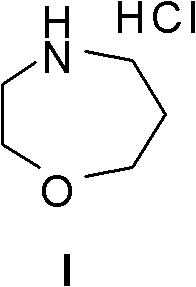
Method for preparing high morphine hydrochloride
A technology of homomorpholine hydrochloride and compounds, applied in the direction of organic chemistry, etc., can solve the problems of unfavorable industrial scale-up production, few literature reports, and low total yield, and achieve high purity of target products, convenient purification, and simplified experimental operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
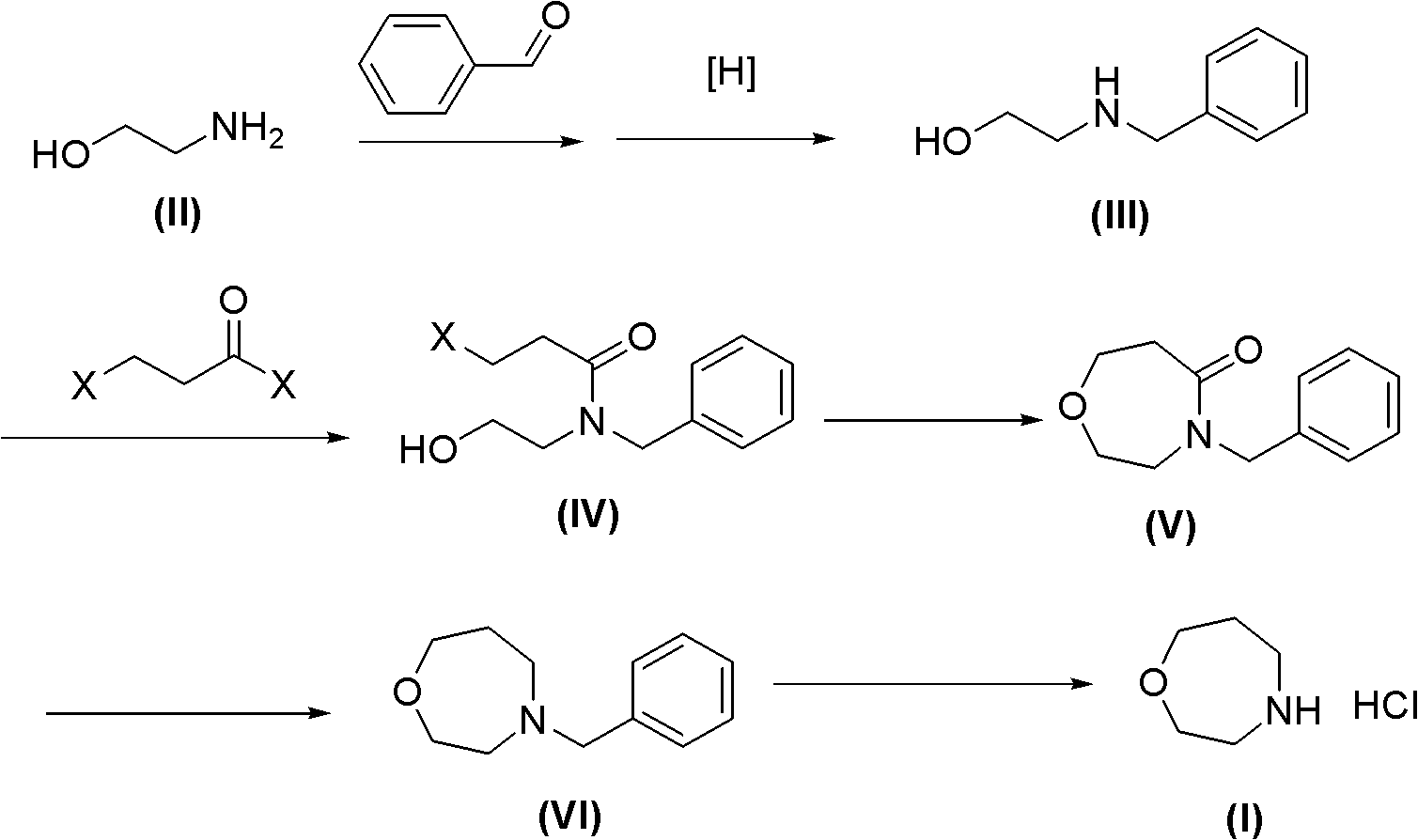
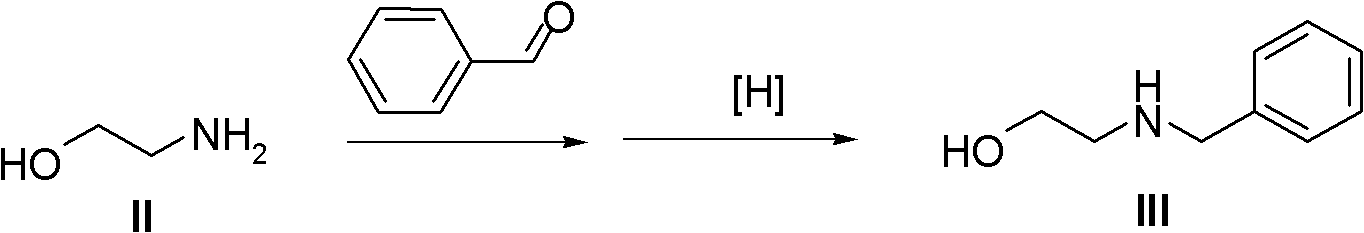
[0038] The synthesis of embodiment one N-benzyl ethanolamine III
[0039] Mix 2.3 kg of ethanolamine, 3.3 kg of benzaldehyde, and 10 kg of methanol in a reaction flask, then add 3.1 kg of sodium triacetate borohydride, heat up to 40°C, and react for 5 hours. The solvent benzaldehyde was removed under reduced pressure, and the obtained compound III was directly used in the next reaction without further purification.
Embodiment 2
[0040] The synthesis of embodiment two N-chloropropionyl-N-benzyl ethanolamine IV
[0041] The above crude compound III and 3 kg of triethylamine were dissolved in 15 liters of dichloromethane. Under ice bath, 5.1 kg of chloropropionyl chloride was slowly added dropwise, and reacted at room temperature for 6 hours after the drop was completed.
[0042] The reaction system was washed with 12 liters of water, the organic layer was dried over anhydrous sodium sulfate, and the organic solvent was removed to obtain 4.8 kg of compound IV. used directly in the next reaction.
Embodiment 3
[0043] The synthesis of embodiment three N-benzyl homomorpholin-2-ketones (V)
[0044] The above 4.8 kg of compound IV was dissolved in 40 liters of tetrahydrofuran. Under an ice bath, 1.5 kg of 50% sodium hydride was added in portions. Then react at room temperature for 2 hours.
[0045] Under ice-cooling, 25 liters of water was added to the reaction mixture to quench the reaction, and extracted three times with 5 liters of ethyl acetate. After the organic layer was dried over anhydrous sodium sulfate, the organic solvent was removed to obtain 3.6 kg of compound V. used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com