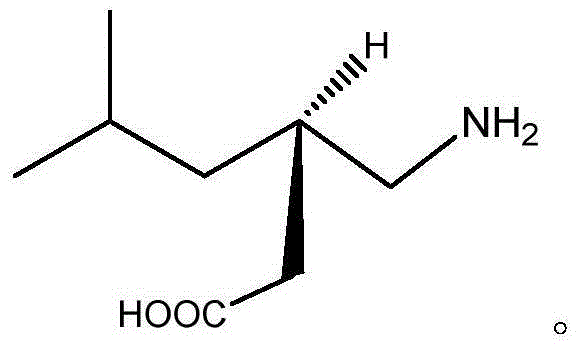
Method for synthesizing pregabalin
A synthesis method and technology of pregabalin, applied in the field of pregabalin, can solve the problems of low total yield of pregabalin, difficult source of raw materials, low product purity, etc., achieve simple reaction route, ensure total yield and purity , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
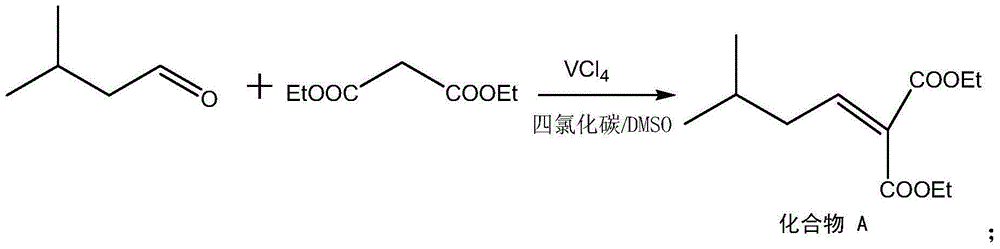
[0028] In the reaction vessel, put isovaleraldehyde, 0.8% vanadium tetrachloride (the total mass of isovaleraldehyde and diethyl malonate is 100%), 2 volumes of DMSO and carbon tetrachloride mixed solvent ( The volume of cyclohexanone is 1), and the mixture is cooled to -5°C. Then add 1.05% of diethyl malonate (based on the amount of isovaleraldehyde as 1), keep the temperature at 5°C, let it react for 12h under constant stirring, filter, distill, and wash with ether to obtain 2-carboxyethyl Base-5-methyl-2-hexenoic acid ethyl ester, this was named compound A.
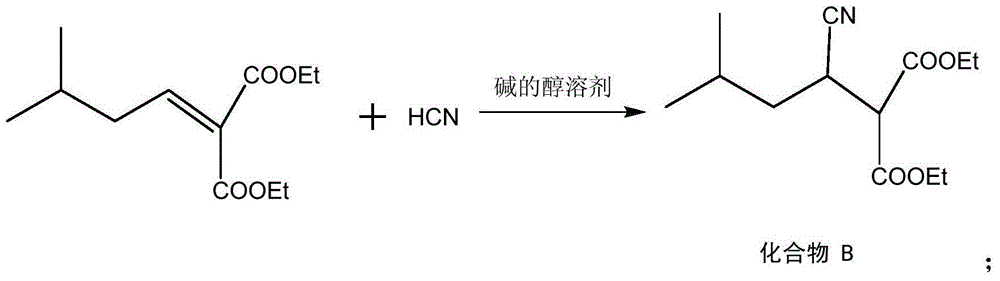
[0029] Put the above-mentioned compound A and ethanol with a mass of 3 (based on the mass of compound A as 1) in a high-pressure reactor, adjust the temperature to 20° C., and slowly drop in HCN with a mass of 1.02 (according to the mass of compound A) The mass is 1 meter). After reacting for 4 hours, the temperature was lowered to 10°C and the solid was filtered out. The solid was washed twice with water, and recry...
Embodiment 2
[0034] In the reaction vessel, put isovaleraldehyde, 4% vanadium tetrachloride (the total mass of isovaleraldehyde and diethyl malonate is 100%), 10 volumes of DMSO and carbon tetrachloride mixed solvent ( The volume of isovaleraldehyde is 1), and the mixture is cooled to 10°C. Then add 1.14 mass of diethyl malonate (based on the mass of isovaleraldehyde as 1), keep the temperature at 10°C, react for 6 hours under constant stirring, filter, distill, and wash with ether to obtain 1,1- Diethyl cyclohexyl allenoate, this was named compound A.
[0035]Put the above-mentioned compound A and methanol with a mass of 10 (based on the mass of compound A as 1) in a high-pressure reactor, adjust the temperature to 35 ° C, and slowly drop in HCN with a mass of 1.08 (calculated as the mass of compound A) The mass is 1 meter). After reacting for 2 hours, the temperature was lowered to 10°C and the solid was filtered out. The solid was washed twice with water, and recrystallized using a m...
Embodiment 3
[0040] In the reaction vessel, put isovaleraldehyde, 2.4% vanadium tetrachloride (the total mass of isovaleraldehyde and diethyl malonate is 100%), 6 volumes of DMSO and carbon tetrachloride mixed solvent ( The volume of isovaleraldehyde is 1), and the mixture is cooled to 7°C. Then add 1.095 mass of diethyl malonate (based on the mass of isovaleraldehyde as 1), keep the temperature at 7°C, react for 9 hours under constant stirring, filter, distill, and wash with ether to obtain 2-carboxyethyl Base-5-methyl-2-hexenoic acid ethyl ester, this was named compound A.
[0041] Put the above-mentioned compound A and ethanol with a mass of 6 (based on the mass of compound A as 1) in a high-pressure reactor, adjust the temperature to 28 ° C, and slowly drop in HCN with a mass of 1.06 (calculated as the mass of compound A) The mass is 1 meter). After reacting for 3 h, the temperature was lowered to 10° C. and the solid was filtered out. The solid was washed twice with water, and recr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com