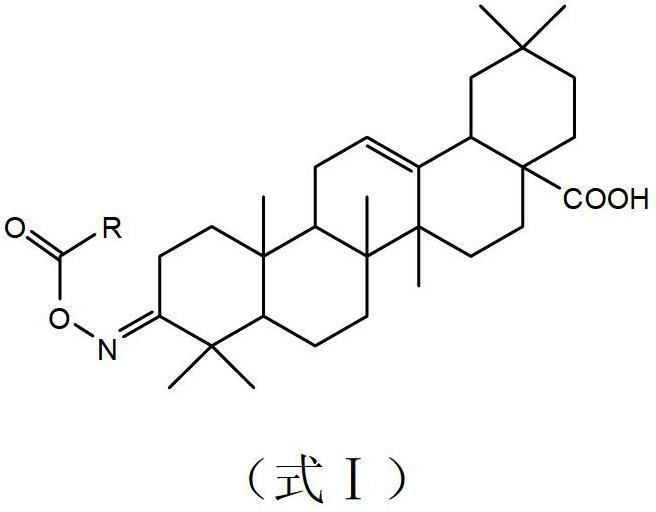
Oleanolic acid oxime ester derivate, preparation method and application thereof
A technology of oxime ester and esterification reaction, applied in the field of oleanolic acid oxime ester derivatives and its preparation and application, to achieve the effects of cheap raw materials, good growth inhibition, and simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Preparation of oleanolic acid oxime protected by benzylation
[0036]
[0037] Add 40g of oleanolic acid and 25g of anhydrous potassium carbonate to a 500mL round bottom flask, dissolve with about 300mL of DMF, and add 16mL of BnBr dropwise. Then react at room temperature for 4h. The system was filtered, the filtrate was washed with water, extracted with dichloromethane, separated, the organic phase was dried and concentrated to obtain 46 g of a white solid. The obtained product was dissolved in anhydrous dichloromethane, 25g of PDC and 25mL of acetic anhydride were added, and heated to reflux for 3h. Precipitate under reduced pressure, dissolve with ethyl acetate, filter with diatomaceous earth layer, concentrate the filtrate to obtain 44g of solid, dissolve with 300mL of dry pyridine, add 53g of hydroxylamine hydrochloride, react at 80°C for 30min, precipitate under reduced pressure, dichloromethane Extraction, washing with water, liquid separation, drying of the...
Embodiment 1
[0038] Example 1. Preparation and structure identification of compound CAU2012-A-01 (R=4-Cl-phenyl).
[0039]
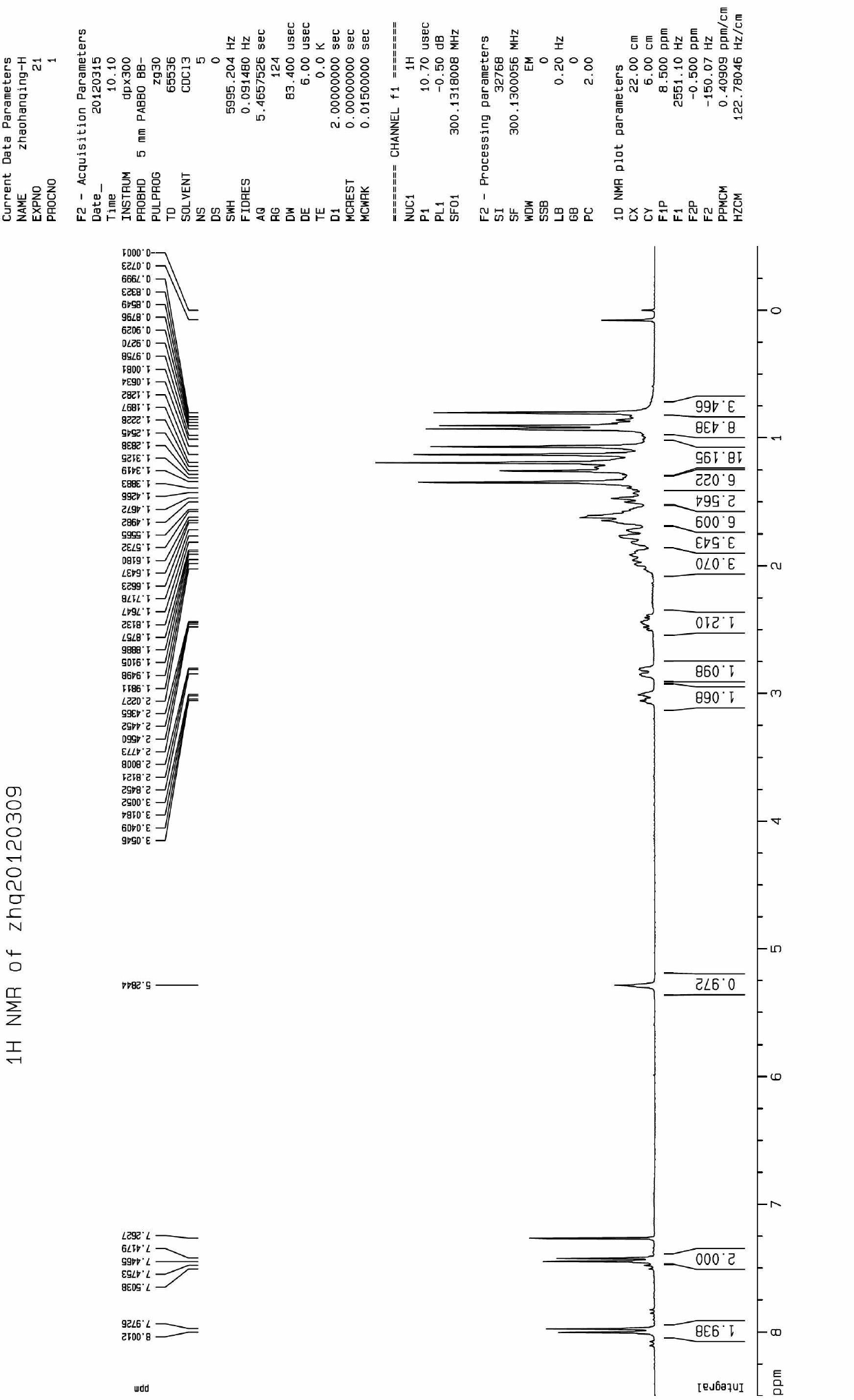
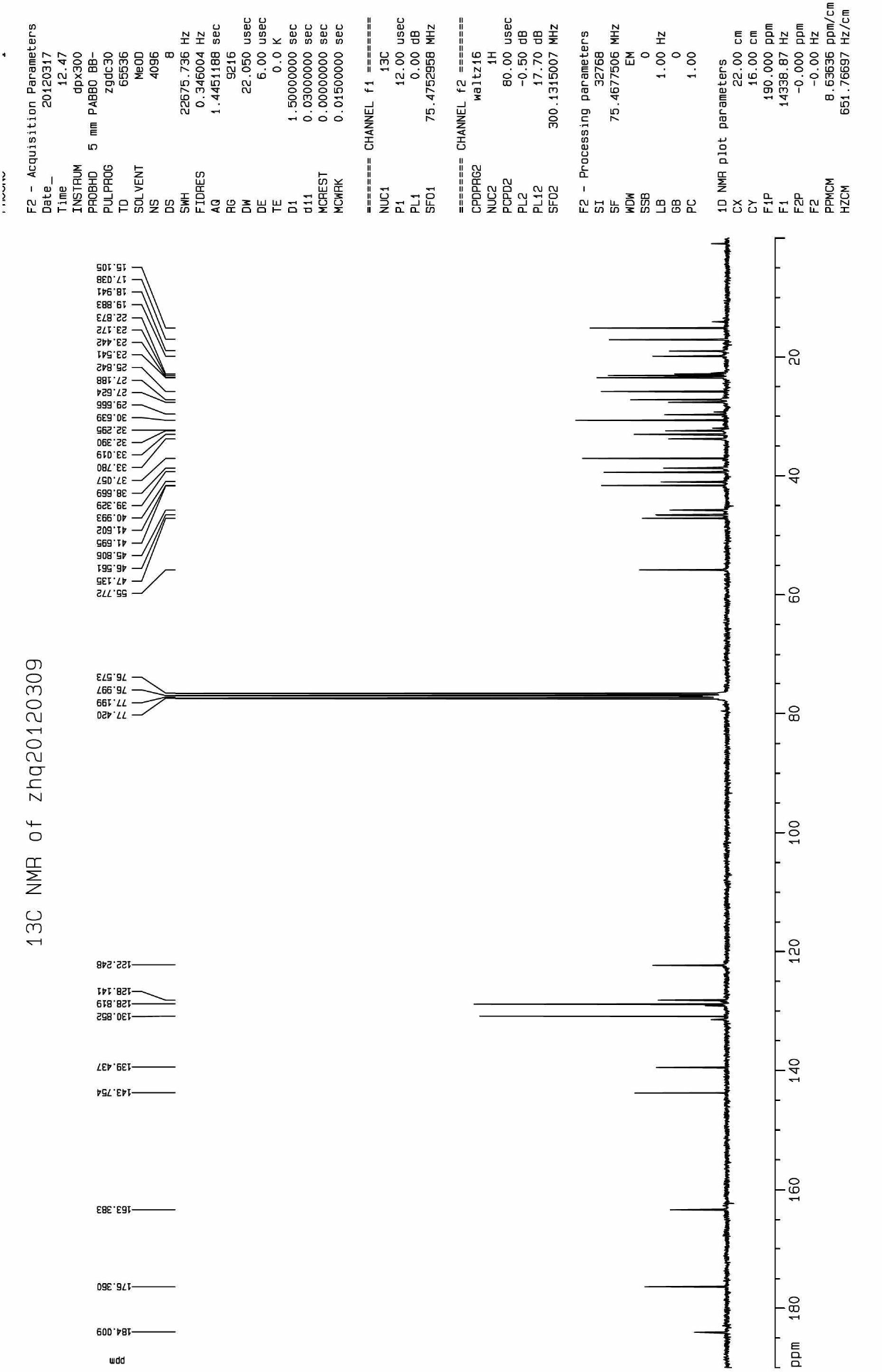
[0040] In a 100mL round-bottomed flask were added benzylated-protected oleanolic acid oxime (2g, 3.5mmol), 4-Cl-benzoic acid (0.66g, 4.2mmol) and DCC (N,N'-dicyclohexylcarbodi imine) (1g, 5mmol), dissolved in 30mL of anhydrous dichloromethane, heated to reflux for 4h, cooled to room temperature after the reaction, filtered, washed with dichloromethane, the filtrate was precipitated under reduced pressure, separated by column chromatography to obtain a white powder solid. The obtained solid was dissolved in an appropriate amount of methanol, 0.8 g of Pd / C was added, and stirred at room temperature under hydrogen for 12 hours. After the reaction was completed, the Pd / C was removed by filtration, and the filtrate was precipitated under reduced pressure. 01, weight 1.8g. The two-step yield was 83%. m.p.98-100.
[0041] The structural confirmation data are as follo...
Embodiment 2
[0044] Embodiment 2, the preparation of compound CAU2012-A-01 emulsifiable concentrate
[0045] Add 1~10g of compound CAU2012-A-01, 5~15g of emulsifier, and 0.1~1g of penetrant into a 100mL volumetric flask, and then use solvents such as toluene, xylene, etc. to dilute to obtain an emulsifiable concentrate with a content of 1~10%.
[0046] Other emulsifiable concentrates of compounds with the general formula CAU2012-A can be prepared according to the above method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com