Method for preparing dichlorohydrin through glycerinum chlorination under catalysis of HY type molecular sieve
A technology of molecular sieve catalyzing glycerol and dichloropropanol, applied in the direction of introducing halogen preparation, organic chemistry, etc., can solve the problems of difficult separation, difficult recovery of organic acids, large catalyst consumption, etc., and achieves easy operation, easy recovery and reuse, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1.5g HY type molecular sieve (purchased from Shanghai Youxin Molecular Sieve Co., Ltd., SiO 2 / Al 2 o 3 =5) Activate at 400°C for 2 hours, then add it into a 100mL four-neck flask containing 50g of glycerin, and stir to disperse evenly. When the system reaches 110°C, the flow rate of 60mL min into the system -1 HCl gas, reacted for 12h, and the tail gas was condensed and then absorbed by NaOH solution to remove HCl.
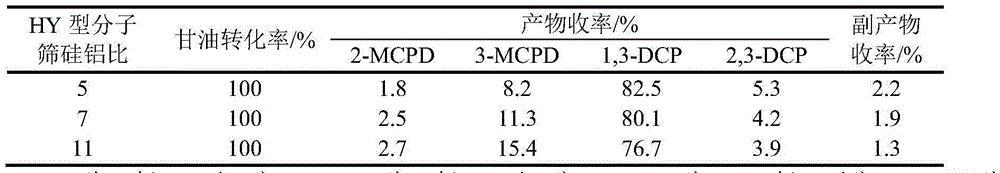
[0018] The molecular sieves in the above steps were changed to HY-type molecular sieves with silicon-aluminum ratios of 7 and 11 respectively, and the influence of molecular sieves with different silicon-aluminum ratios on the chlorination reaction of glycerin was studied. The experimental results are shown in Table 1:
[0019] Table 1 Effect of molecular sieves with different ratios of silicon to aluminum on the chlorination of glycerin
[0020]
[0021] Note: 2-MCPD is 2-chloro-1,3-propanediol, 3-MCPD is 3-chloro-1,2-propanediol, 1,3-DCP is 1,3-di...
Embodiment 2
[0024] 0.1g HY type molecular sieve (purchased from Shanghai Youxin Molecular Sieve Co., Ltd., SiO 2 / Al 2 o 3 =5) Activate at 400°C for 2 hours, then add it into a 100mL four-neck flask containing 50g of glycerin, and stir to disperse evenly. When the system reaches 110°C, the flow rate of 60mL min into the system -1 HCl gas, reacted for 12h, and the tail gas was condensed and then absorbed by NaOH solution to remove HCl.
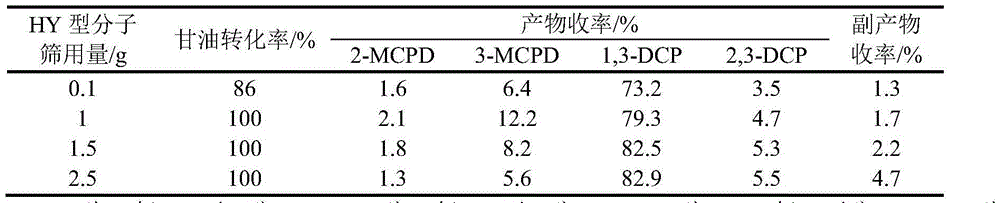
[0025] Change the amount of molecular sieve in the above steps to 1g, 1.5g and 2.5g, and study the influence of catalyst amount on the experiment, the results are shown in Table 2:
[0026] Table 2 Effect of catalyst dosage on the chlorination reaction of glycerol
[0027]
[0028] Note: 2-MCPD is 2-chloro-1,3-propanediol, 3-MCPD is 3-chloro-1,2-propanediol, 1,3-DCP is 1,3-dichloro-2-propanol, 2, 3-DCP is 2,3-dichloro-1-propanol.
[0029] It can be seen from Table 2 that with the increase of the amount of catalyst, the reaction rate increases, but...
Embodiment 3
[0031] 1.5g HY type molecular sieve (purchased from Shanghai Youxin Molecular Sieve Co., Ltd., SiO 2 / Al 2 o 3 =5) Activate at 400°C for 2 hours, then add it into a 100mL four-neck flask containing 50g of glycerin, and stir to disperse evenly. When the system reaches 110°C, the flow rate of 60mL min into the system -1 HCl gas, reacted for 12h, and the tail gas was condensed and then absorbed by NaOH solution to remove HCl.
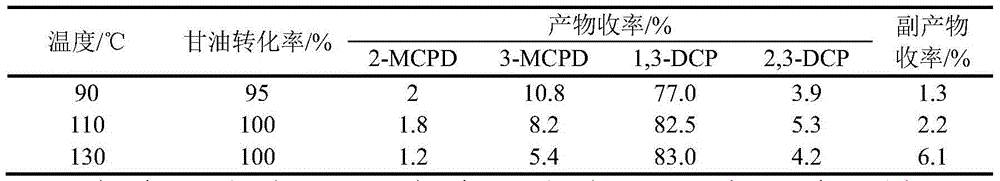
[0032] The reaction temperature in the above steps was changed to 90°C and 130°C to study the effect of the reaction temperature on the chlorination reaction of glycerol. The experimental results are shown in Table 3:
[0033] Table 3 Effect of reaction temperature on the chlorination reaction of glycerol
[0034]
[0035] Note: 2-MCPD is 2-chloro-1,3-propanediol, 3-MCPD is 3-chloro-1,2-propanediol, 1,3-DCP is 1,3-dichloro-2-propanol, 2, 3-DCP is 2,3-dichloro-1-propanol.
[0036] With the increase of reaction temperature, the rate of glycerin chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com