Cephaene onium salt compound and its preparation, and synthesis of cephapyrazde sulfate therefrom
A compound, cephem technology, applied in chemical instruments and methods, preparation of lactams, organic chemistry, etc., can solve the problem of expensive reagents and other problems, and achieve the effects of easy disposal of three wastes, easy recovery, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
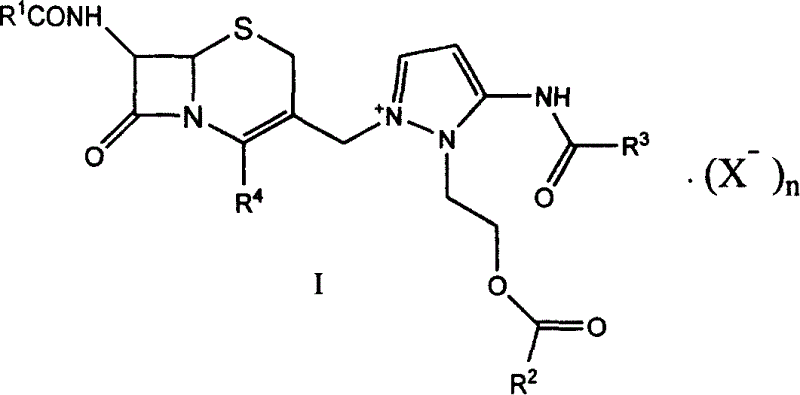
[0046] 7β-formylamino-3-[3-formylamino-2-(2-formyloxyethyl)-1-pyrazolium]methyl-3-cephem-4-carboxylate (I-a) Preparation of:
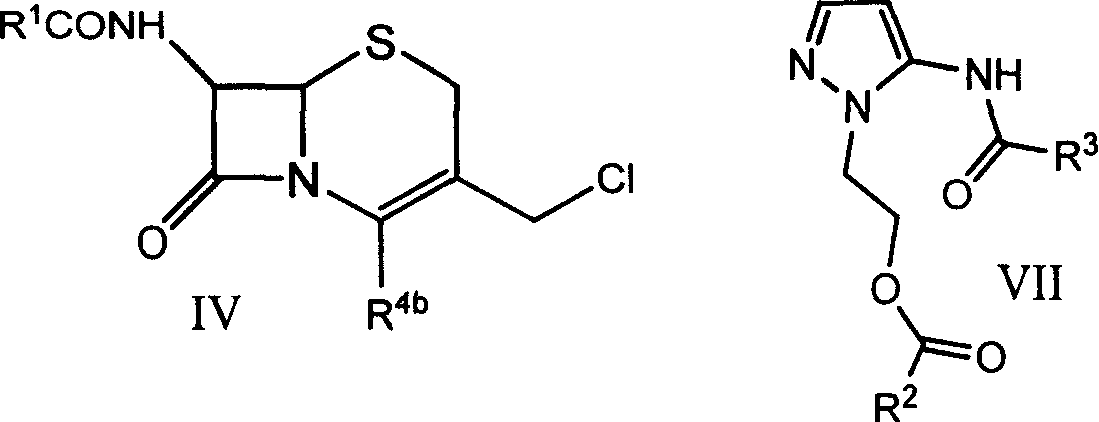
[0047] In a 250ml reaction flask, add compound 7β-formamido-3-chloromethyl-3-cephem-4-carboxylic acid (IV-a) 50g, dimethylformamide 200ml, sodium bicarbonate 30g, and 5-Formylamino-1-(2-formyloxyethyl)pyrazole (VIII) 99g, stirred at room temperature for 5 hours, filtered, poured the filtrate into 2000ml of ethyl acetate, filtered, collected the resulting precipitate, 75 g of light yellow solid compound (I-a) was obtained.
[0048] IR (KBr, Max): 3423, 1770, 1632, 1574cm -1
[0049] m / e: 424 [M-H] +
Embodiment 2
[0051] 7β-acetylamino-3-[3-formylamino-2-(2-formyloxyethyl)-1-pyrazolium]methyl-3-cephem-4-carboxylate (I-b) preparation:
[0052] Add 20g of 7β-acetylamino-3-chloromethyl-3-cephem-4-carboxylic acid, 40ml of sulfolane, 7.2g of sodium carbonate, and 5-formylamino-1-(2-methyl Acyloxyethyl) pyrazole (VII) 15.5g, stirred at room temperature for 2 hours, filtered, the filtrate was poured into 800ml acetone, filtered, and the resulting precipitate was collected to obtain 29g of light yellow solid compound (I-b).
[0053] IR (KBr, cm -1 ):
Embodiment 3
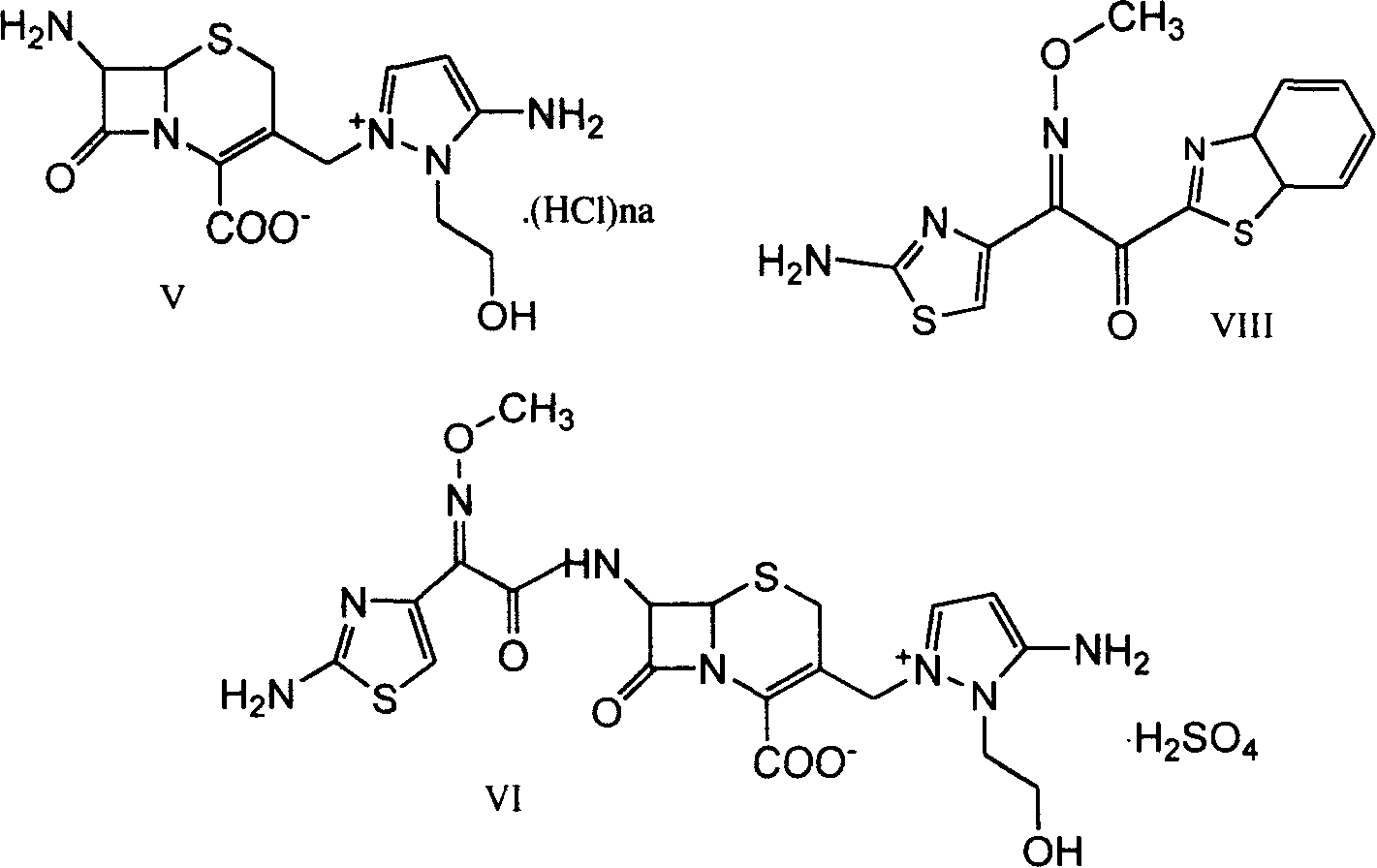
[0055] Preparation of 7β-amino-3-[3-amino-2-(2-hydroxyethyl)-1-pyrazolium]methyl-3-cephem-4-carboxylic acid chloride hydrochloride (V) :
[0056] At room temperature, add 23ml of concentrated hydrochloric acid to compound 7β-formylamino-3-[3-formylamino-2-(2-formyloxyethyl)-1-pyrazolium]methyl-3-cephalosporin In the mixture of ene-4-carboxylate (I-a) 20g and methanol 100ml, at the same temperature, after stirring for 2 hours, the mixture was added dropwise into 200ml of ethyl acetate, and a nearly white solid was precipitated, filtered, and an appropriate amount of ethyl acetate After ester washing, 17 g of white solid compound (V) was obtained.
[0057] 1 H-NMR (D 2 O) δ: 3.16-3.31 (6H, m), 3.57-3.60 (2H, t), 4.09-4.37 (2H, m),
[0058] 4.85(1H, d), 5.01(1H, d). 5.06-5.28(2H, g), 5.87(1H, d),
[0059] 7.38(2H, s), 8.00(1H, d)
[0060] IR (KBr, cm -1 ): 3413, 1786, 1636, 1592
[0061] m / e: 340 [M-H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com