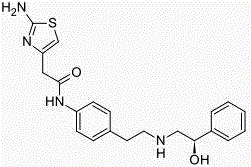
Mirabegron medicine composition and preparation method thereof
A technology of mirabegron and its composition, which is applied in the field of mirabegron pharmaceutical composition and its preparation, can solve problems such as easy moisture absorption, unstable preparation, unstable sustained release, etc., to reduce product production cost, product The effect of stable quality and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
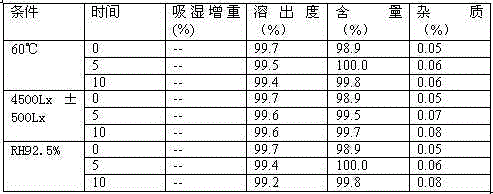
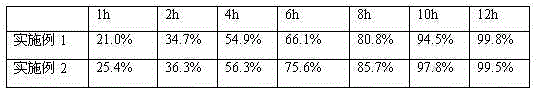
Image
Examples
Embodiment 1
[0044] Every 1000 described mirabegron pharmaceutical compositions, its formula consists of:
[0045] Mirabegron 25g
[0046] Hydroxypropyl methylcellulose 125g
[0047] Mannitol 62.5g
[0048] Microcrystalline cellulose 20g
[0049] Magnesium stearate 7.5g
[0050] Micropowder silica gel 5g
[0051] Preparation process: 1) Pulverize mirabegron, hydroxypropyl methylcellulose, mannitol and microcrystalline cellulose through a 100-mesh sieve respectively, and set aside;
[0052] 2) Weigh the above-mentioned materials in the prescription amount, make soft materials with 10% povidone K30 absolute ethanol solution, and granulate with 20 mesh;
[0053] 3) Ventilate and dry in a drying oven for 2-3 hours at a temperature of 50±2°C;
[0054] 4) Add magnesium stearate and micropowder silica gel, and mix well with dry granules;
[0055] 5) Calculate the actual tablet weight, adjust the tablet weight, and press the tablet;
[0056] 6) Prepare the prescribed amount of film coating...
Embodiment 2
[0059] Every 1000 described mirabegron pharmaceutical compositions, its formula consists of:
[0060] Mirabegron 50g
[0061] Ethylcellulose 133.3g
[0062] Mannitol 66.7g
[0063] Microcrystalline cellulose 66.7g
[0065] Micropowder silica gel 6.7g
[0066] Preparation process: 1) Pulverize mirabegron, ethyl cellulose, mannitol and microcrystalline cellulose through a 100-mesh sieve respectively, and set aside;
[0067] 2) Weigh the above-mentioned materials in the prescription amount, make soft materials with 10% povidone K30 absolute ethanol solution, and granulate with 20 mesh;
[0068] 3) Ventilate and dry in a drying oven for 2-3 hours at a temperature of 50±2°C;
[0069] 4) Add magnesium stearate and micropowder silica gel, and mix well with dry granules;
[0070] 5) Calculate the actual tablet weight, adjust the tablet weight, and press the tablet;
[0071] 6) Prepare the prescribed amount of film coating agent with 70% ethanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com