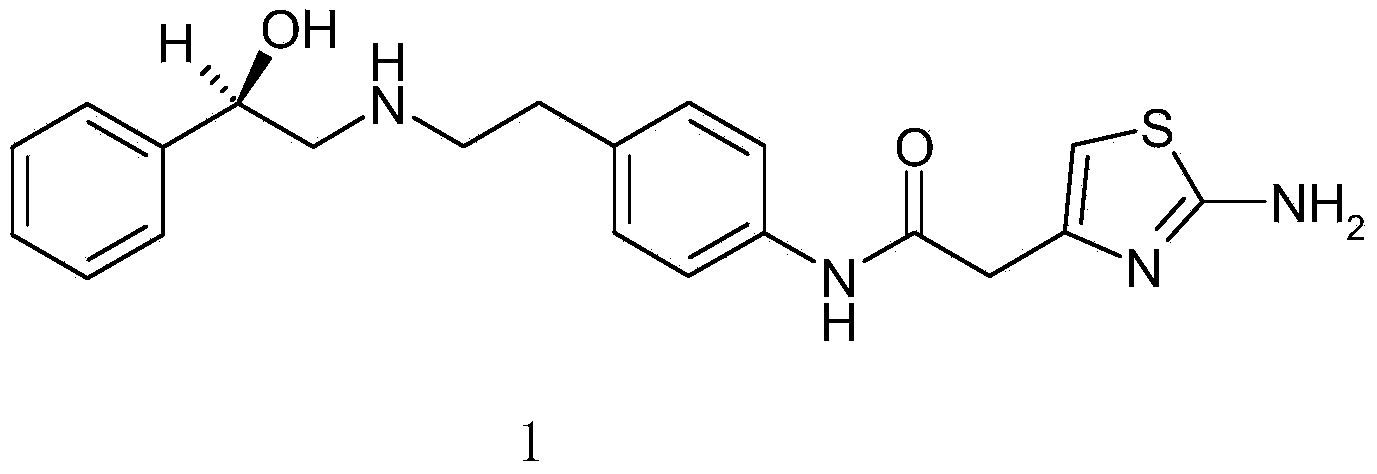
Preparation method of mirabegron
A technology of structural formula and compound, which is applied in the field of preparation of mirabegron, can solve the problems of high cost, low yield of synthetic mirabegron, unsuitability for industrial production, etc., and achieves low environmental pollution, convenient yield and purity, The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
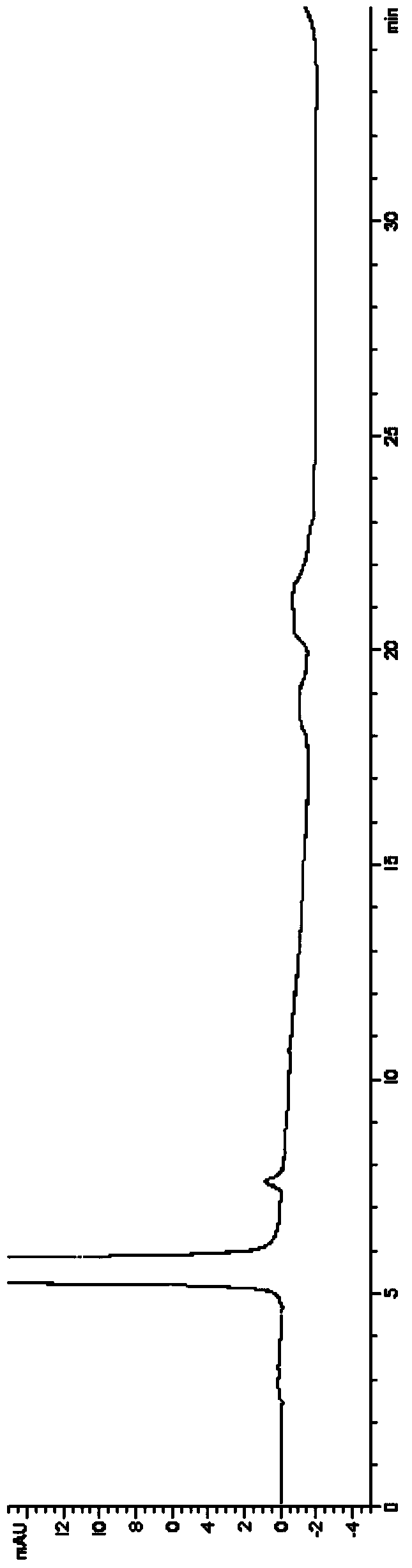
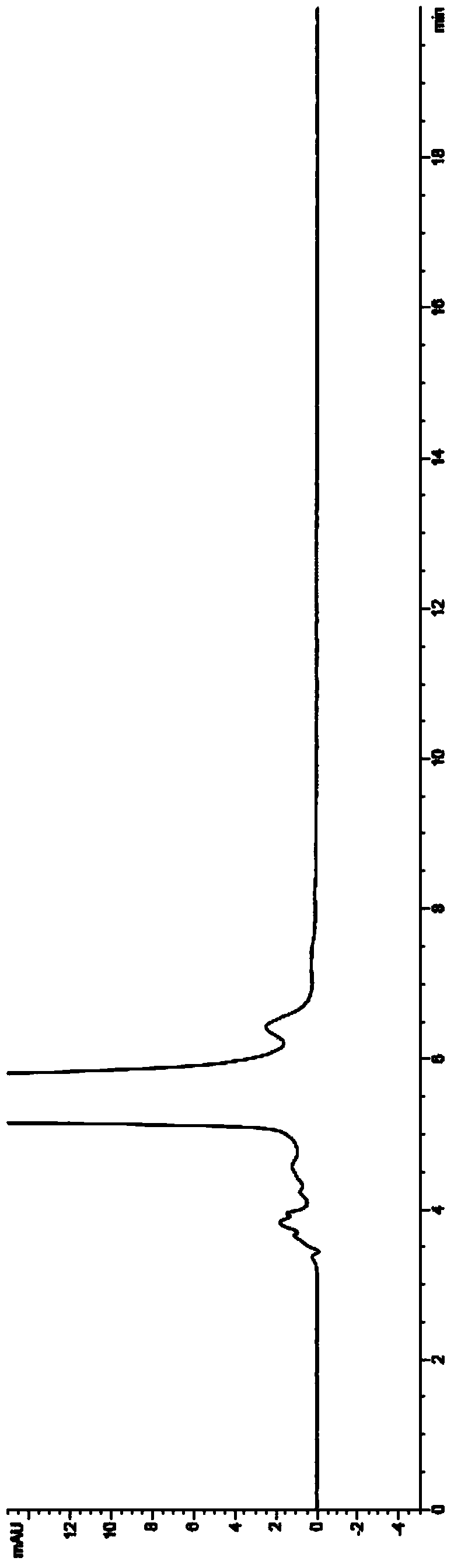
Image
Examples
Embodiment 1
[0050] In a 1000ml reaction flask, put 2-(2-aminothiazole-4-)acetic acid hydrochloride (50g, 0.26mol), triethylamine (39g, 0.39mol), di-tert-butyl dicarbonate (61.6g, 0.28mol), ethanol 500ml, reacted at 20-30°C for 12 hours, recovered the solvent under reduced pressure, added 200ml of water, 100ml of ethyl acetate, extracted and separated layers, washed the organic layer once with 100ml of saturated saline, and concentrated to dryness under reduced pressure to obtain 63g of the compound of structural formula 4, yield 95%.
[0051] In a 2000ml reaction bottle, drop into the compound of the above structural formula 4 (60g, 0.23mol), the compound of the structural formula 3 (54.7g, 0.23mol) substituted by bromine, 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (44.5g, 0.23mol), 1-hydroxybenzotriazole (31.4g, 0.23mol), triethylamine (58.7g, 0.58mol), dichloromethane 700ml, 20-30℃ After 12 hours of reaction, 500ml of water and 100ml of dichloromethane were added, a...
Embodiment 2
[0056] In a 1000ml reaction flask, add 2-(2-aminothiazole-4-)acetic acid hydrochloric acid (50g, 0.26mol), triethylamine (64.9g, 0.64mol), benzyl bromide (44g, 0.28mol), methanol 500ml , reacted at 20-30°C for 10 hours, recovered the solvent under reduced pressure, added 200ml of water, 100ml of ethyl acetate, extracted and separated layers, washed the organic layer once with 100ml of saturated brine, concentrated to dryness under reduced pressure, and obtained 61.6g of the compound of structural formula 4 , yield 93%.
[0057] In a 2000ml reaction bottle, drop into the compound of the above structural formula 4 (60g, 0.24mol), the compound of the structural formula 3 (56.9g, 0.24mol) substituted by bromine, 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (46.3g, 0.24mol), 1-hydroxybenzotriazole (32.7g, 0.24mol), triethylamine (61.1g, 0.60mol), dichloromethane 700ml, 30-40℃ After reacting for 12 hours, add 500ml of water and 100ml of dichloromethane, extract an...
Embodiment 3
[0062] In a 1000ml reaction flask, add 2-(2-aminothiazole-4-)acetic acid hydrochloride (50g, 0.26mol), triethylamine (64.9g, 0.64mol), benyl chloroformate (48.2g, 0.28mol), 500ml of ethyl acetate was reacted at 20-30°C for 10 hours, 200ml of water and 100ml of ethyl acetate were added, the layers were extracted, the organic layer was washed once with 100ml of saturated brine, concentrated under reduced pressure to dryness, and 67.6g of the compound of structural formula 4 was obtained. The yield is 90%.
[0063] In a 2000ml reaction bottle, put the compound of the above structural formula 4 (60g, 0.21mol), the compound of the structural formula 3 (44.2g, 0.23mol) substituted by chlorine, N,N-carbonyldiimidazole (39.9g, 0.25mol), triethyl Amine (31.1g, 0.31mol), ethyl acetate 700ml, react at 40-50°C for 4 hours, add chlorine-substituted compound of structural formula 3 (37.3g, 0.24mol), react at 40-50°C for 8 hours, add water 500ml , 100ml of ethyl acetate was extracted and se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com