Mirabegron sustained-release pharmaceutical composition
A technology for mirabegron and sustained-release tablets, which is applied in the field of pharmaceutical preparations, can solve the problems of high price of polyethylene oxide and increase the cost of pharmaceutical manufacturing, and achieves the guarantee of drug efficacy and safety, excellent release duration, and guaranteed The effect of medication safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] prescription composition
[0023]
[0024]
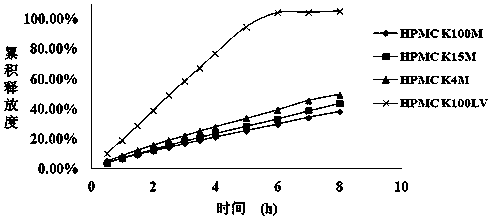
[0025] The total HPMC viscosity (20°C, 2% aqueous solution) of the skeleton material in the prescription is 120mPa·S.
[0026] Preparation:
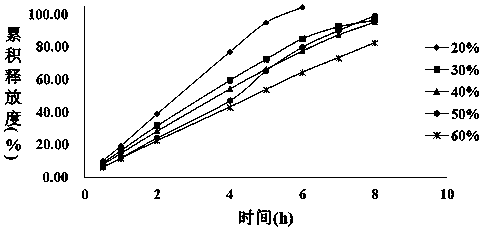
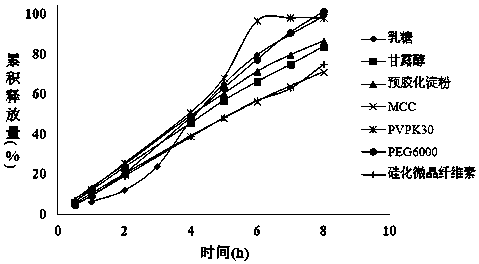
[0027] With 1000g mirabegron crude drug, 330g HPMC K4MCR , 1670g HPMC K100LV Add 960g PEG6000 and 790g lactose into the fluidized bed granulator, granulate with 200g 10% HPC-SL aqueous solution, after granulation, add 25g magnesium stearate and 25g micropowder silica gel, press with rotary tablet press Tablets to obtain Mirabegron sustained-release tablets with a tablet weight of 250 mg.
[0028] Dissolution test:
[0029] Dissolution test was carried out to the prepared Mirabegron sustained-release tablets according to USP dissolution test method (basket method). Use 900mL of pH 6.8 phosphate buffer as the release medium, and use UV to measure the cumulative release at 2h, 4h, and 8h. The results are as follows:
[0030] time 2h 4h 8h cumulative release (...
Embodiment 2
[0040] prescription form
[0041]
[0042] The total HPMC viscosity (20°C, 2% aqueous solution) of the skeleton material in the prescription is 115mPa·S.
[0043] Preparation:
[0044] With 1000g mirabegron crude drug, 330g HPMC K4MCR 、HPMC K100LV 1670, 960g polyethylene glycol and 790g lactose were added to the fluidized bed granulator, granulated with 200g 10% HPC-SL aqueous solution, after granulation, 25g magnesium stearate and 25g micropowder silica gel were added, and the Tablet machine carries out tabletting, adopts high-efficiency coating machine to the prepared tablet, sprays the aqueous dispersion of coating film coating agent (Opadry 03F22733), obtains the Mirabegron sustained-release tablet of tablet weight 257.5mg.
Embodiment 3
[0046] prescription form
[0047]
[0048]
[0049] The total HPMC viscosity (20°C, 2% aqueous solution) of the skeleton material in the prescription is 85mPa·S.
[0050] Preparation:
[0051] With 100g mirabegron bulk drug, HPMC K4MCR 38g, HPMC K100LV 187g, PEG6000155g, placed in a mixing granulator to disperse evenly, adding 15g of 10% hydroxypropyl cellulose aqueous solution for granulation, after granulation, adding 5g of magnesium stearate and fully mixing, using a rotary tablet press for tableting, Obtain the mirabegron sustained-release tablet of tablet weight 250mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com