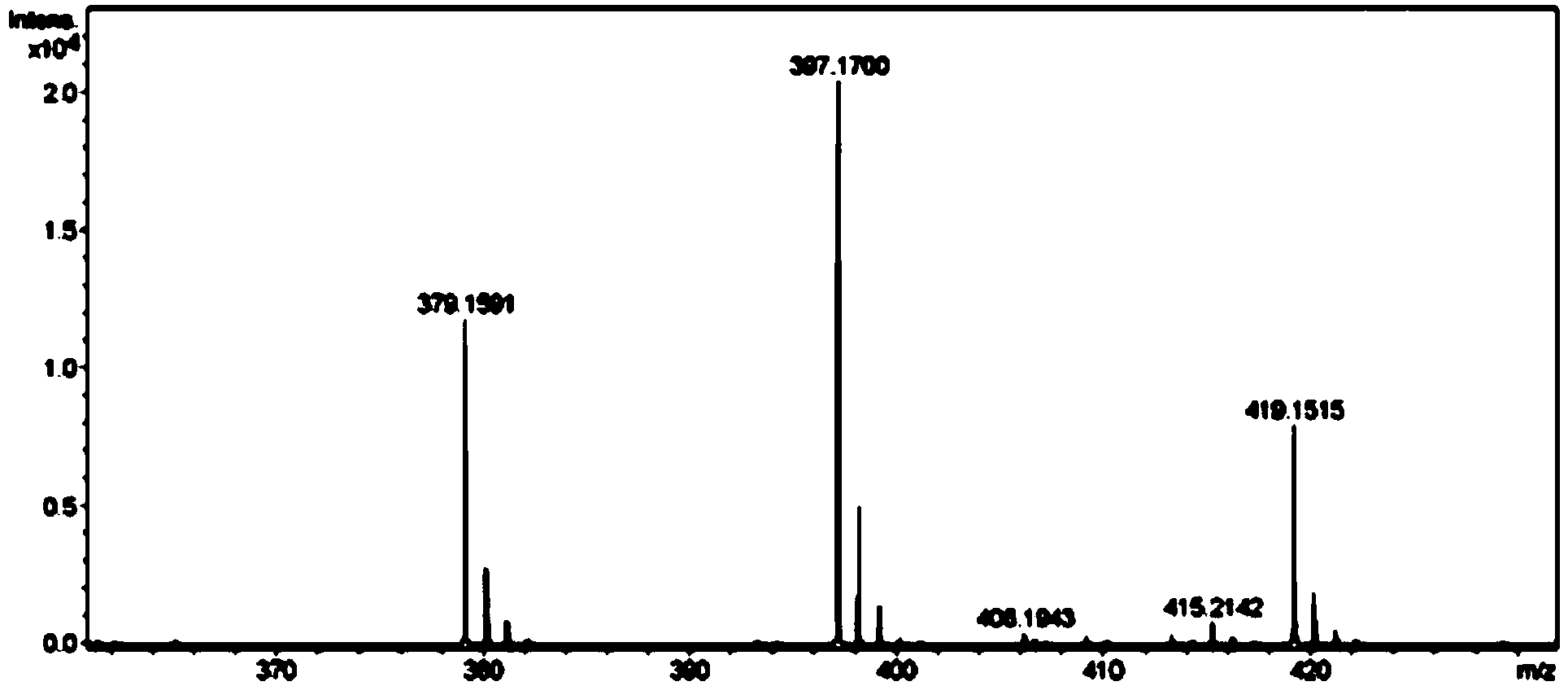
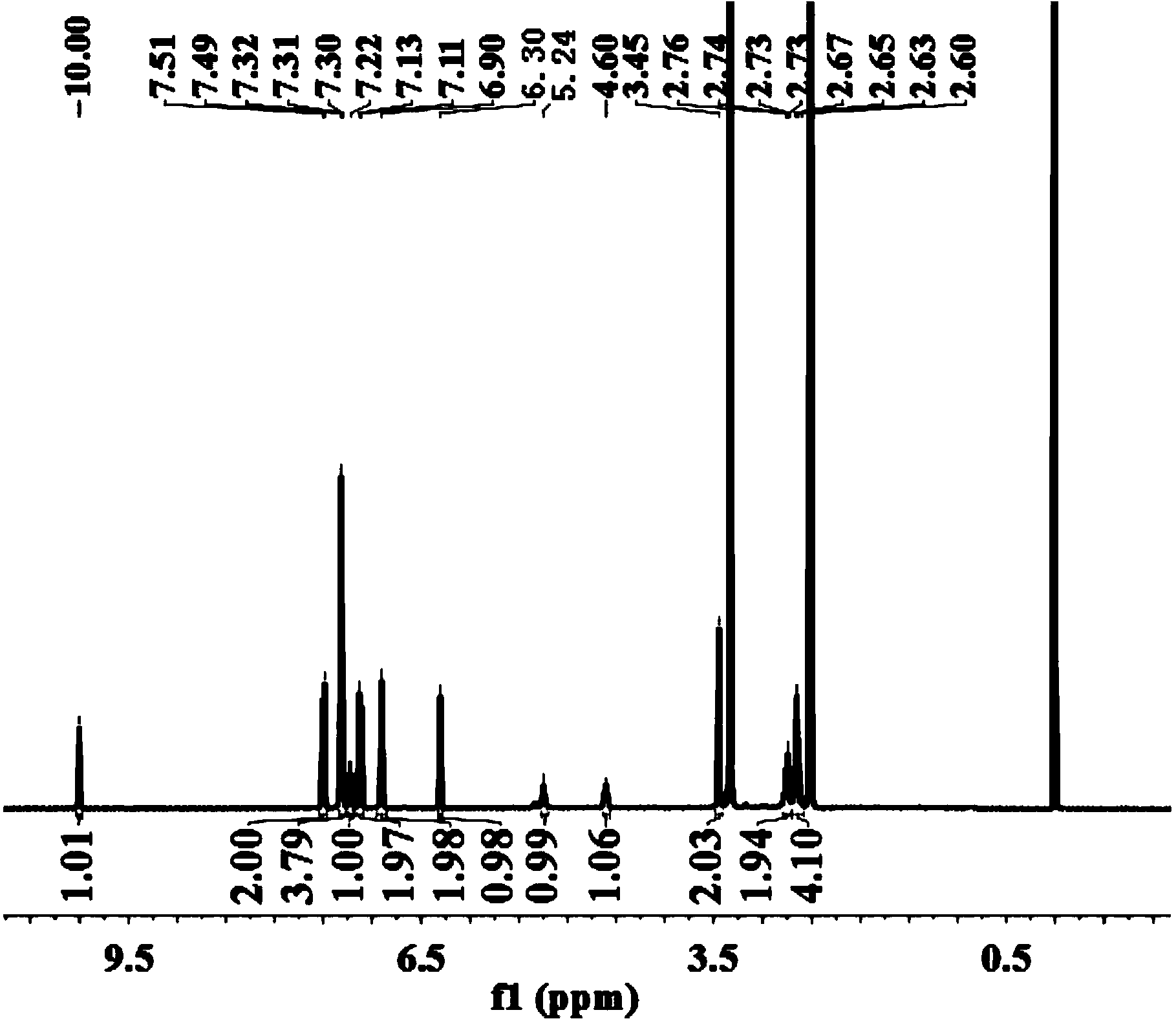
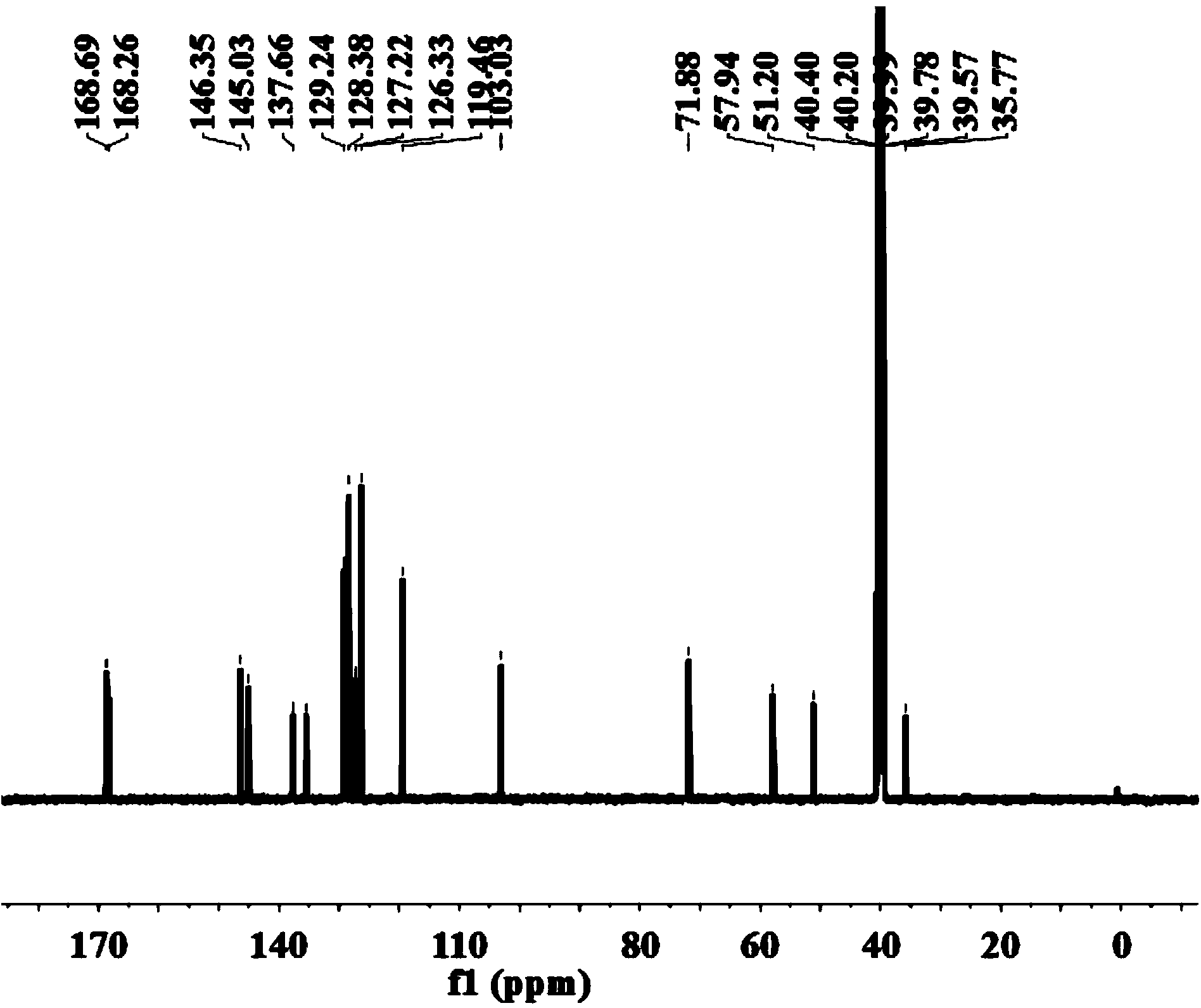
Method for synthesizing mirabegron
A synthesis method and solvent technology, applied in the field of chemical drug synthesis, can solve the problems of non-scale-up production, complex post-processing, unfriendly environment, etc., and achieve the effect of convenient post-processing, fewer reaction steps, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0025] Specific embodiment one: the synthetic method of Mirabegron of the present embodiment, carry out according to the following steps:
[0026] 1. Add nitrophenylethylamine and (R)-styrene oxide into the solvent according to the molar ratio of 1:(1.0~1.5), and carry out the ring-opening reaction at 60°C~70°C. After the reaction is completed, Then recrystallize with a recrystallization solvent to obtain (R)-2-((4-nitrophenethyl)amino)-1-phenethyl-1-alcohol;
[0027] Two, the (R)-2-(4-nitrophenethyl) amino)-1-phenethyl-1-alcohol obtained in step 1 is added in the solvent, and then according to the catalyst and (R)-2-(( The molar ratio of 4-nitrophenethyl)amino)-1-phenethyl-1-alcohol is (0.01~0.05): 1, add the catalyst, carry out the reduction reaction at room temperature and hydrogen atmosphere, and wait for the reaction to complete Afterwards, the solvent is evaporated under reduced pressure to obtain (R)-2-((4-aminophenyl)amino)-1-phenethyl-1-alcohol; wherein the solvent i...
specific Embodiment approach 2
[0032] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the solvent described in step 1 is isopropanol, ethyl acetate, dichloromethane, tetrahydrofuran or acetonitrile. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0033] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the ring-opening reaction is carried out at 60° C. to 65° C. in step 1. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com