HPLC analysis method of mirabegron-related substances
An analysis method and related substance technology, applied in the field of drug analysis of aryl ethanolamine β3 receptor agonists, can solve the problems of incomplete detection and interference of impurity detection, and achieve fast analysis speed, reliable impurity control, and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Detection method:
[0054]Take an appropriate amount of Mirabegron test sample, weigh it accurately, add 30% methanol to dissolve and dilute it into a sample solution of 1 mg / ml, shake it up, and accurately measure 20 μl and inject it into the liquid chromatograph. Perform HPLC analysis at 30°C and record the chromatograms.
[0055] The concentration of potassium dihydrogen phosphate buffer in mobile phase A is 0.02mol / L, the pH is 5.5, and the ratio of methanol is 12%.
[0056] Analyze the system suitability solution according to the following gradient elution procedure
[0057] time (min) Mobile phase A% Mobile phase B% 0 100 0 5 100 0 40 50 50 50 50 50 51 100 0 60 100 0
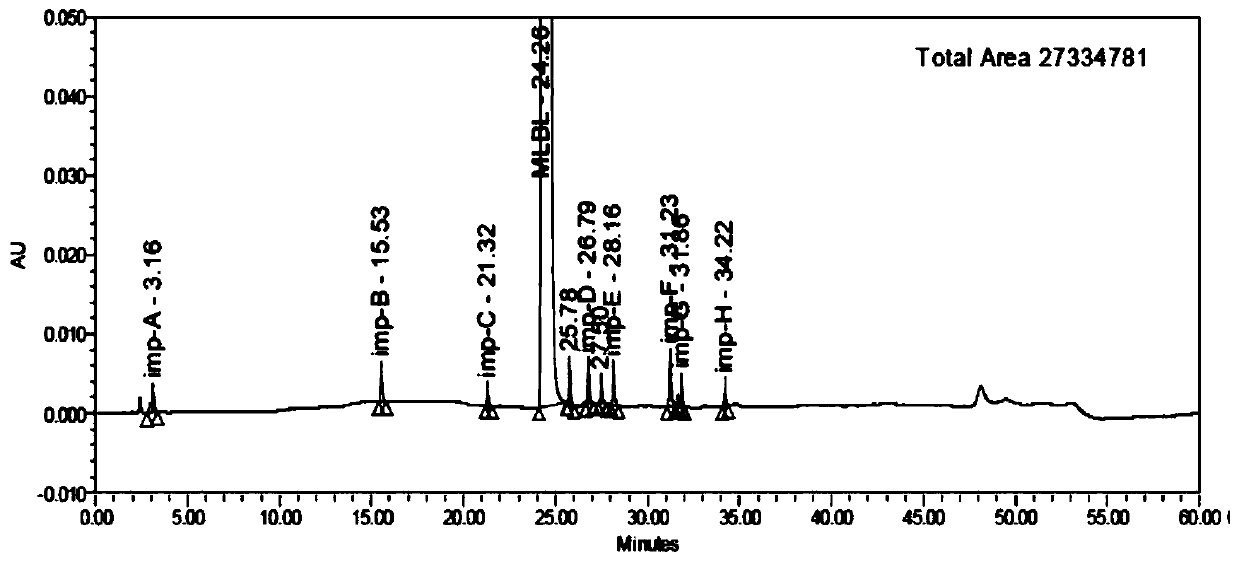
[0058] Chromatogram see figure 1 , the retention time of each impurity and the main peak and the resolution between adjacent peaks are shown in Table 1.
[0059] Table 1
[0060]
[0061]
[0062] From the data in Table 1, it can be se...
Embodiment 2
[0064] Others are the same as embodiment 1, the difference is:
[0065] The concentration of potassium dihydrogen phosphate buffer in mobile phase A is 0.01mol / L or 0.05mol / L;
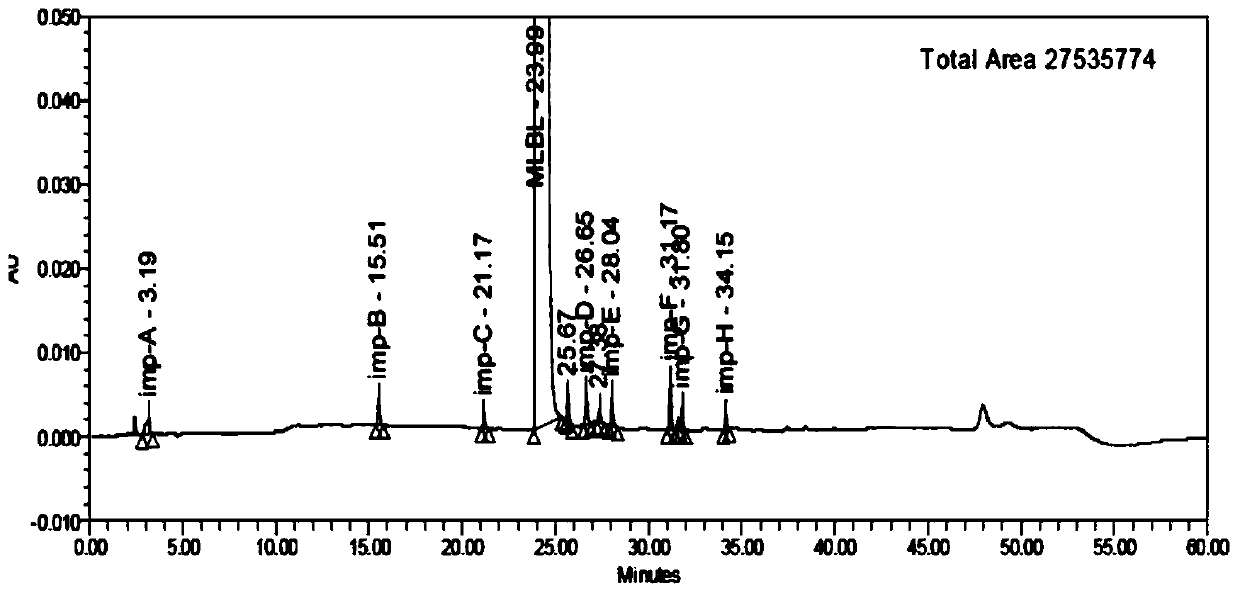
[0066] Analyze the system adaptability solution according to the gradient elution program in Example 1, see the chromatogram figure 2 with 3 , the retention time of each impurity and the main peak and the resolution between adjacent peaks are shown in Table 2 and Table 3.
[0067] When the concentration of potassium dihydrogen phosphate buffer solution in mobile phase A in table 2 is 0.01mol / L, the result of resolution
[0068]
[0069]
[0070] When the concentration of potassium dihydrogen phosphate buffer solution in mobile phase A in table 3 is 0.05mol / L, the result of resolution
[0071] name / code retention time (min) Resolution between adjacent peaks Impurity A 3.187 / Impurity B 15.583 42.14 Impurity C 21.152 34.26 Mirabegron 24.182 11.40 ...
Embodiment 3
[0075] Others are the same as embodiment 1, the difference is:
[0076] The pH of potassium dihydrogen phosphate buffer in mobile phase A is 4.0 or 6.5;
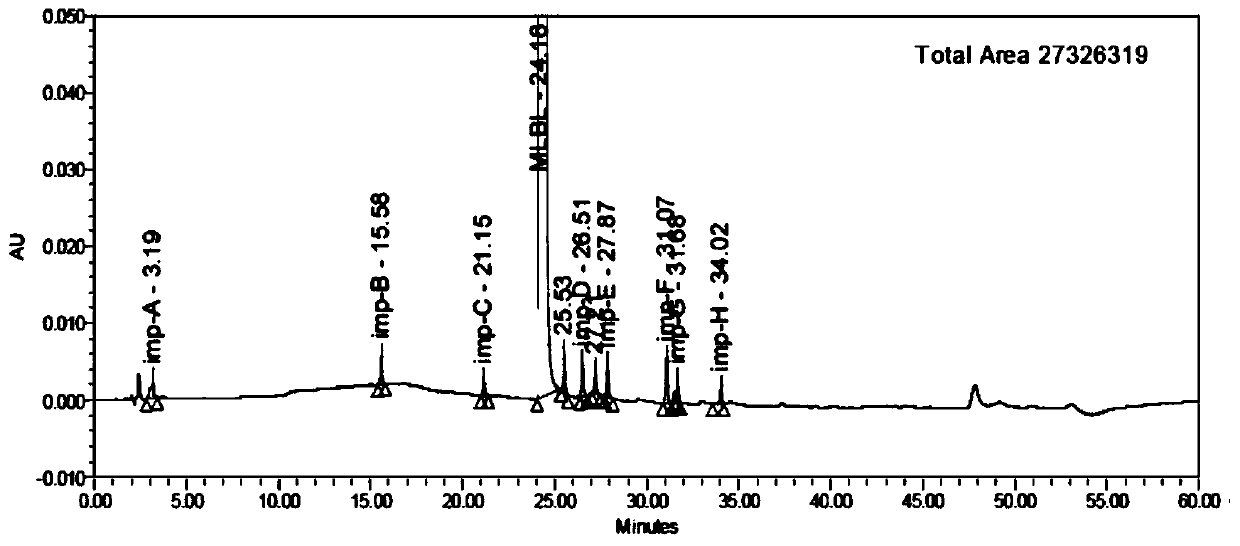
[0077] Analyze the system adaptability solution according to the gradient elution program in Example 1, see the chromatogram Figure 4 with 5 , the retention time of each impurity and the main peak and the resolution between adjacent peaks are shown in Table 4 and Table 5.
[0078] The separation result when the pH of potassium dihydrogen phosphate buffer in table 4 mobile phase A is 4.0
[0079]
[0080]
[0081] The separation result when the pH of potassium dihydrogen phosphate buffer solution in table 5 mobile phase A is 6.5
[0082] name / code retention time (min) Resolution between adjacent peaks Impurity A 3.243 / Impurity B 15.281 39.99 Impurity C 21.927 40.61 Mirabegron 25.165 12.15 Impurity D 27.470 5.85 Impurity E 28.953 3.92 Impurity F 31.375 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com