Preparation method of mirabegron
An amino, phenylethanol technology, applied in the direction of organic chemistry and other directions, can solve the problems of difficult to realize industrialized production, difficult to obtain starting materials, too long synthesis route, etc., to achieve mild and controllable conditions, broad market prospects and industrial application value , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
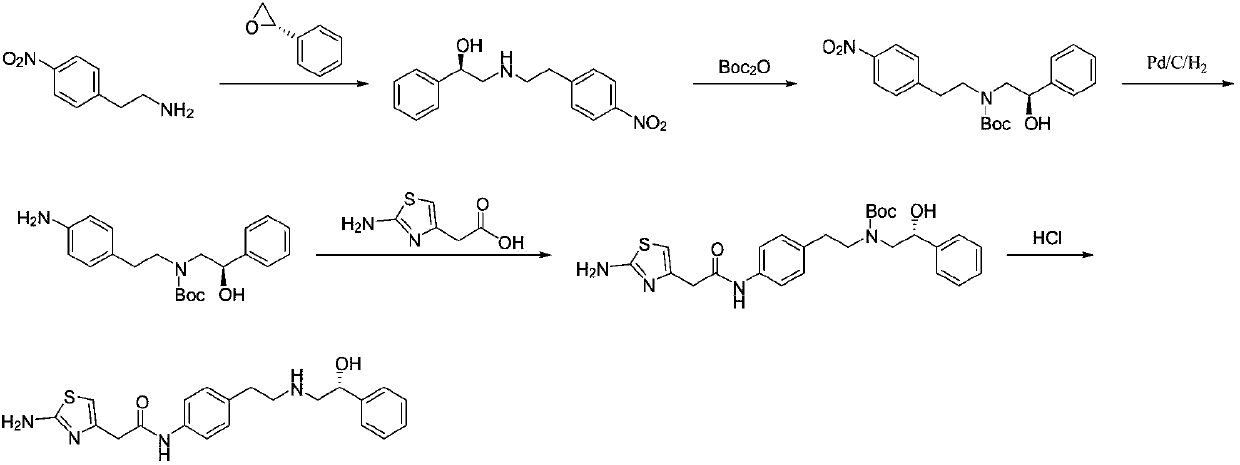
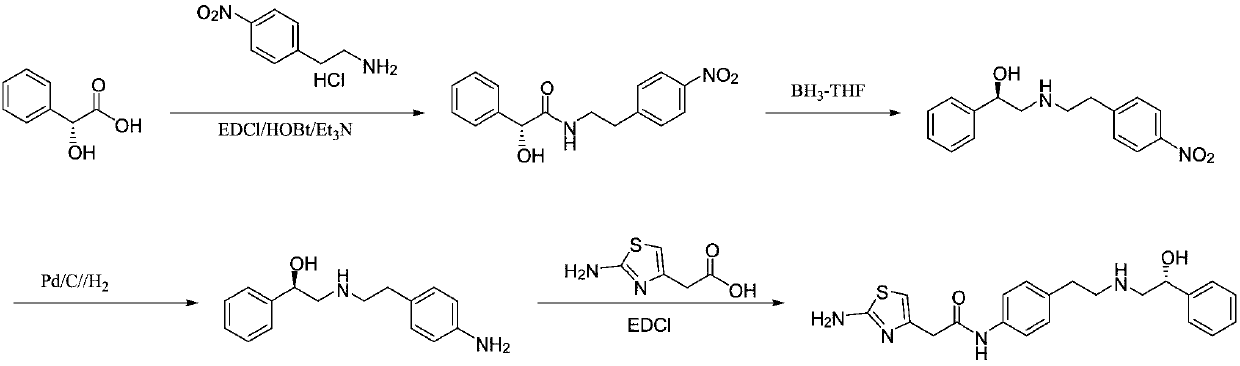
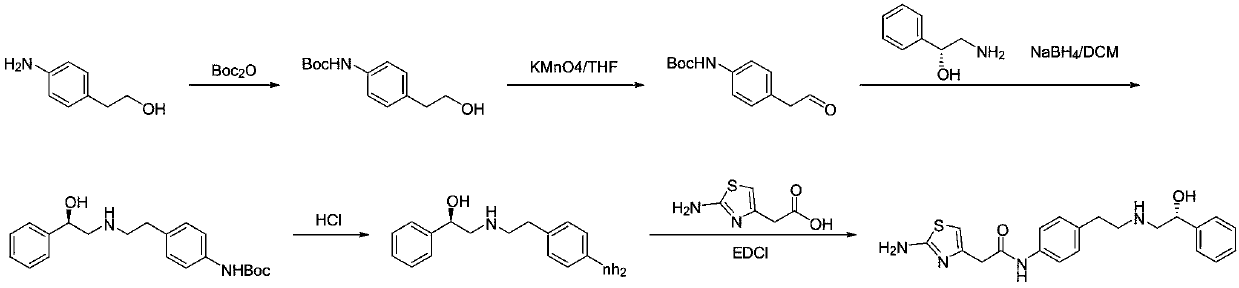
[0038] A kind of preparation method of Mirabegron proposed by the present invention comprises the following steps:
[0039] S1, p-nitrophenylacetonitrile is reduced to obtain p-nitrophenylacetaldehyde;
[0040] S2. Condensation and reduction of p-nitrophenylacetaldehyde and (R)-2-amino-1-phenylethanol to obtain (R)-2-((4-nitrophenylethyl)amino)-1-phenyl ethanol;
[0041] S3. Reducing (R)-2-((4-nitrophenethyl)amino)-1-phenylethanol to obtain the intermediate (R)-2-((4-aminophenethyl)amino )-1-phenylethanol;
[0042] S4. Condensing (R)-2-((4-aminophenethyl)amino)-1-phenylethanol with aminothiazoleacetic acid to obtain Mirabegron.
Embodiment 2
[0044] A kind of preparation method of Mirabegron proposed by the present invention comprises the following steps:
[0045] S1, p-nitrophenylacetonitrile is obtained through reduction reaction with diisobutylaluminum hydride as a reducing agent to p-nitrophenylacetaldehyde; wherein, the molar ratio of diisobutylaluminum hydride to p-nitrophenylacetonitrile is 1.1:1 ;
[0046] S2. With diethylamine and 4-N,N-lutidine as catalysts, p-nitrophenylacetaldehyde and (R)-2-amino-1-phenylethanol are condensed and reduced by potassium borohydride, wherein the condensation The reduction temperature is -78°C to obtain (R)-2-((4-nitrophenethyl)amino)-1-phenylethanol;
[0047] S3. Reducing (R)-2-((4-nitrophenethyl)amino)-1-phenylethanol to obtain the intermediate (R)-2-((4-aminophenethyl)amino )-1-phenylethanol; wherein, the reaction system in the reduction reaction is ammonium formate-Pd / C system; the reduction reaction temperature is 10°C, and the reduction reaction time is 8 hours;
...
Embodiment 3
[0050] A kind of preparation method of Mirabegron proposed by the present invention comprises the following steps:
[0051] S1, p-nitrophenylacetonitrile is obtained through reduction reaction with diisobutylaluminum hydride as a reducing agent to p-nitrophenylacetaldehyde; wherein, the molar ratio of diisobutylaluminum hydride to p-nitrophenylacetonitrile is 1.5:1 ;
[0052] S2. Using pyridine as a catalyst, p-nitrophenylacetaldehyde and (R)-2-amino-1-phenylethanol are condensed and reduced by sodium triacetoxy borohydride, wherein the condensation reduction temperature is 50° C. to obtain (R )-2-((4-nitrophenethyl)amino)-1-phenylethanol;
[0053] S3. Reducing (R)-2-((4-nitrophenethyl)amino)-1-phenylethanol to obtain the intermediate (R)-2-((4-aminophenethyl)amino )-1-phenylethanol; wherein, the reaction system in the reduction reaction is ammonium formate-Pd / C system; the reduction reaction temperature is 65°C, and the reduction reaction time is 2 hours;
[0054] S4. Usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com