Synthetic method of mirabegron intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of cumbersome post-processing steps, unfriendly environment, difficult recycling, etc., and achieve the effect of facilitating large-scale industrial production, high feasibility of industrial operation, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
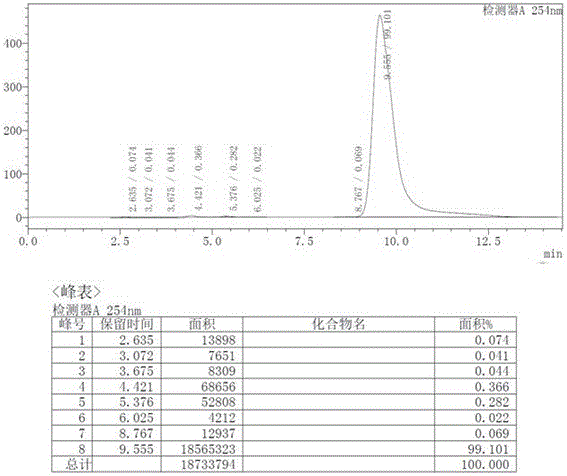
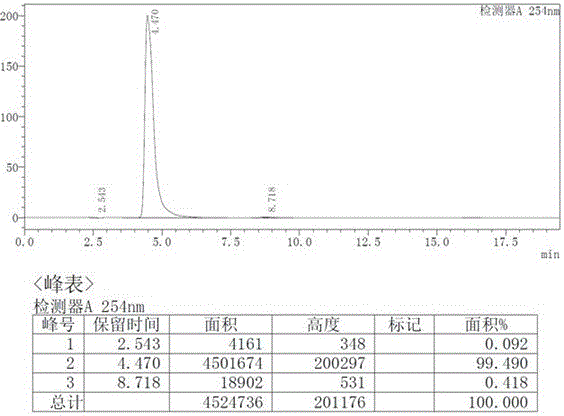
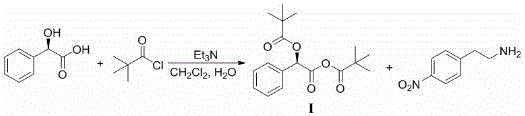
[0028] Preparation of intermediate (I): Add R-mandelic acid (12.17g, 80mmol, 1eq), triethylamine (23.28mL, 168mmol, 2.1eq) and dichloromethane (100mL ). Under ice bath, slowly add pivaloyl chloride (19.29g, 160mmol, 2.0eq) dropwise, keep the temperature of the reaction system not exceeding 5°C, after the dropwise addition is completed, rise to room temperature, add water (15mL) and slowly raise the temperature to 60°C for reaction After 3h, the reaction was completed and cooled to room temperature to obtain the mixed anhydride intermediate (I).
[0029] Preparation of intermediate (II): at room temperature, slowly add 4-nitrophenethylamine (12.61g, 76mmol, 0.95eq) dropwise to the dichloromethane / water solution of the above-mentioned mixed anhydride intermediate (I), dropwise After the addition was completed, the reaction was carried out at room temperature for 2h. After the reaction is completed, wash with 1 mol / L dilute hydrochloric acid (20 mL), separate layers, concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com