Mirabegron sustained release tablet and preparation method thereof
A technology of mirabegron and sustained-release tablets, applied in the field of mirabegron sustained-release tablets and its preparation, can solve the problems of cumbersome granulation operations and the release of sustained-release components of mirabegron sustained-release tablets, etc. Achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This embodiment relates to a Mirabegron sustained-release tablet and its preparation method:
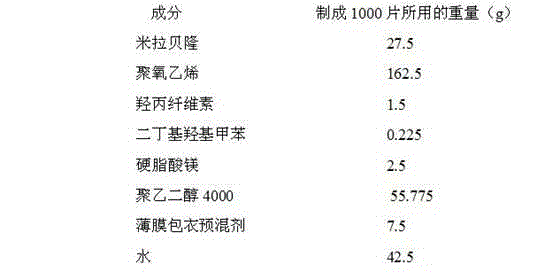
[0024] The composition ratio of Mirabegron sustained-release tablets is:
[0025]
[0026] (The composition of the film coating premix is hydroxypropyl methylcellulose, iron oxide red, and titanium dioxide; since water is removed during drying, it does not appear in the final product of the formulation)
[0027] The preparation method includes the following steps:
[0028] Step one, raw material pretreatment: take Mirabegron in a mortar and grind and smash it through a 100-mesh sieve. Dibutyl hydroxytoluene, hydroxypropyl cellulose and magnesium stearate are respectively passed through a 100-mesh sieve. The ethylene glycol is passed through a 60 mesh sieve;
[0029] Step 2: Add the pre-treated polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose to the pre-treated Mirabegron according to the above-mentioned dosage ratio, and pass through a 60-mesh sieve 6 times and mix it...
Embodiment 2
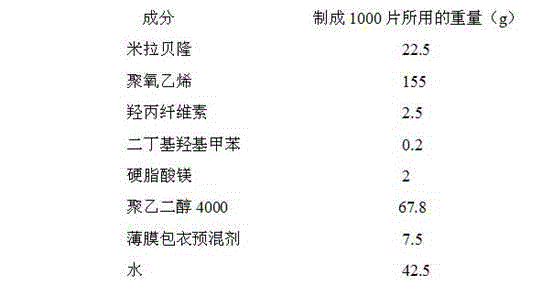
[0033] The composition ratio of Mirabegron sustained-release tablets is:
[0034]
[0035] (The composition of the film coating premix is hydroxypropyl methylcellulose, iron oxide red, and titanium dioxide; since water is removed during drying, it does not appear in the final product of the formulation)
[0036] The preparation method includes the following steps:
[0037] Step one, raw material pretreatment: take Mirabegron in a mortar and grind and smash it through a 100-mesh sieve. Dibutyl hydroxytoluene, hydroxypropyl cellulose and magnesium stearate are respectively passed through a 100-mesh sieve. The ethylene glycol is passed through a 60 mesh sieve;
[0038] Step 2: Add the pre-treated polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose to the pre-treated Mirabegron according to the above-mentioned dosage ratio, and pass through a 60-mesh sieve 6 times and mix it evenly as a premix;
[0039] Step 3: Add the pre-treated dibutyl hydroxytoluene to the premix accordi...
Embodiment 3
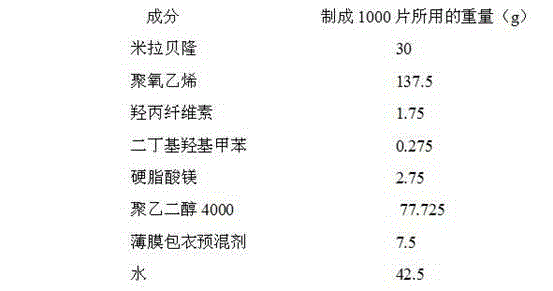
[0042] The composition ratio of Mirabegron sustained-release tablets is:
[0043]
[0044] (The composition of the film coating premix is hydroxypropyl methylcellulose, iron oxide red, and titanium dioxide; since water is removed during drying, it does not appear in the final product of the formulation)
[0045] The preparation method includes the following steps:
[0046] Step one, raw material pretreatment: take Mirabegron in a mortar and grind and smash it through a 100-mesh sieve. Dibutyl hydroxytoluene, hydroxypropyl cellulose and magnesium stearate are respectively passed through a 100-mesh sieve. The ethylene glycol is passed through a 60 mesh sieve;
[0047] Step 2: Add the pre-treated polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose to the pre-treated Mirabegron according to the above-mentioned dosage ratio, and pass through a 60-mesh sieve 6 times and mix it evenly as a premix;
[0048] Step 3: Add the pre-treated dibutyl hydroxytoluene to the premix accordi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com