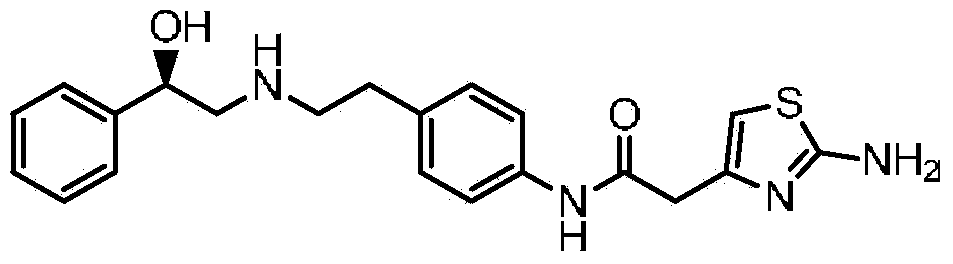
Acetylaniline compounds and application thereof in preparation of mirabegron
A technology of phenylacetamide and compound, applied in the new preparation field of mirabegron, can solve the problems of unstable aldehyde properties, difficult to control, difficult and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
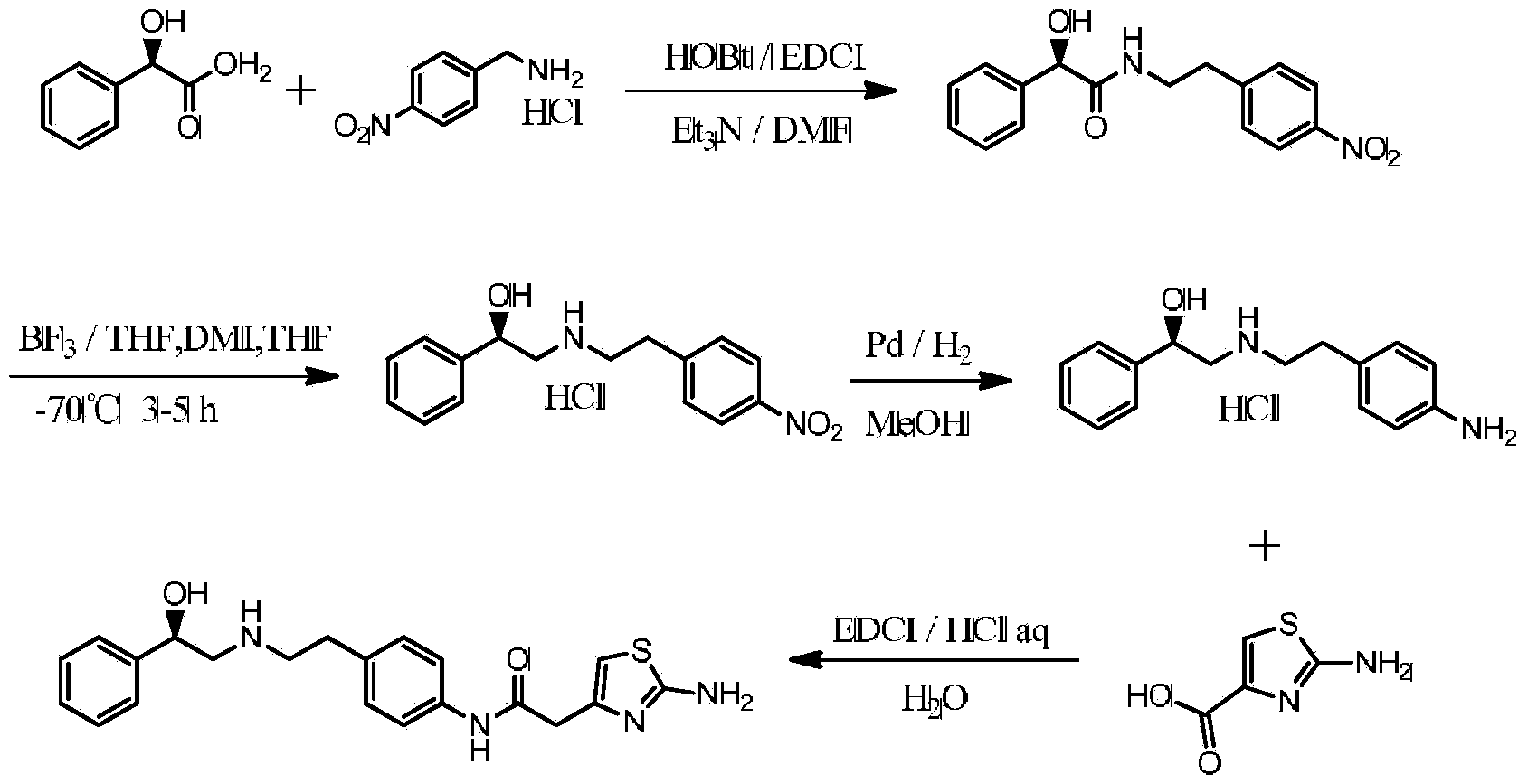
[0034] 1. Preparation of (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide
[0035] Add 141g (0.93mol) of (R)-2-hydroxy-2-phenylacetic acid, 180g (0.95mol) of (4-nitrophenyl)methylamine hydrochloride, and 187g (1.86mol) of triethylamine into the reaction flask , DMF 700ml, then add 125g hydroxybenzotriazole (0.93mol), EDCI 178g (0.93mol), then stir the reaction at room temperature for 5h, add 2L water to the reaction solution, add ethyl acetate to extract 3 times, and combine the organic layers , washed 3 times with water, then the organic layer was concentrated to dryness, the residue was recrystallized with 1L toluene, filtered, and dried in vacuo to obtain yellow crystals (R)-N-(4-nitrophenethyl)-2-hydroxy- 2-phenylacetamide 249g, yield 89%; FAB-MS (m / z): 301.2(M+H)+
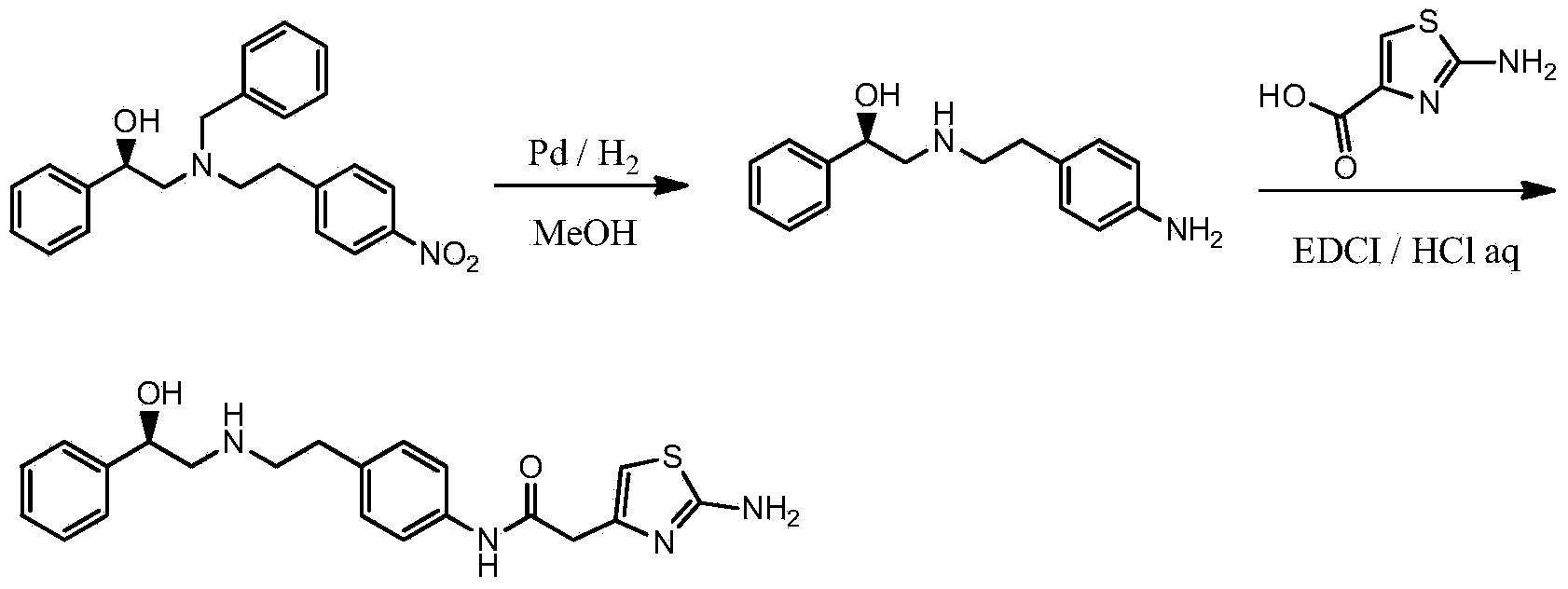
[0036] 2. Preparation of (R)-N-(4-aminophenethyl)-2-hydroxy-2-phenylacetamide
[0037] Add (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide 240g (0.8mol) into the reaction flask, add methanol (1.6L),...
Embodiment 2
[0047] 1. Preparation of (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide
[0048] Add (R)-2-hydroxy-2-phenylacetic acid 90g (0.59mol), (4-nitrophenyl)methylamine hydrochloride 114g (0.60mol), triethylamine 119g (1.18mol) into the reaction flask , DMF 700ml, then add 72g 4-dimethylaminopyridine (0.59mol), dicyclohexylcarbodiimide 122g (0.59mol), then stir the reaction at room temperature for 5h, add 1.4L water to the reaction solution, add ethyl acetate Extracted 3 times, combined the organic layers, washed 3 times with water, then concentrated the organic layer to dryness, the residue was recrystallized with 0.64L toluene, filtered, and dried in vacuo to obtain yellow crystals (R)-N-(4-nitrobenzene Ethyl)-2-hydroxy-2-phenylacetamide 137g, yield 77%; FAB-MS (m / z): 301.2(M+H)+
[0049] 2. Preparation of (R)-N-(4-aminophenethyl)-2-hydroxy-2-phenylacetamide
[0050] Add (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide 125g (0.4mol) into the reaction flask, add methanol ...
Embodiment 3
[0060] 1. Preparation of (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide
[0061] Add (R)-2-hydroxy-2-phenylacetic acid 100g (0.66mol), (4-nitrophenyl)methylamine hydrochloride 126g (0.67mol), triethylamine 133g (1.32mol) into the reaction flask , DMF 600ml, then add 90g 1-hydroxy-7-azobenzotriazole (0.66mol), DIC 83g (0.66mol), then stir the reaction at room temperature for 5h, add 1.5L water to the reaction solution, add ethyl acetate The ester was extracted 3 times, the organic layers were combined and washed 3 times with water, then the organic layer was concentrated to dryness, the residue was recrystallized with 0.7L toluene, filtered, and dried in vacuo to obtain yellow crystals (R)-N-(4-nitro Phenylethyl)-2-hydroxy-2-phenylacetamide 156g, yield 79%; FAB-MS (m / z): 301.2(M+H) +
[0062] 2. Preparation of (R)-N-(4-aminophenethyl)-2-hydroxy-2-phenylacetamide
[0063] Add (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide 145g (0.48mol) into the reaction flask, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com