(R)-3-amino piperidine hydrochloride preparation method
A technology of aminopiperidine and hydrochloride, applied in the direction of organic chemistry, can solve the problems of high cost, difficult industrialization, and low yield, and achieve the effects of low production cost, high raw material utilization rate, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
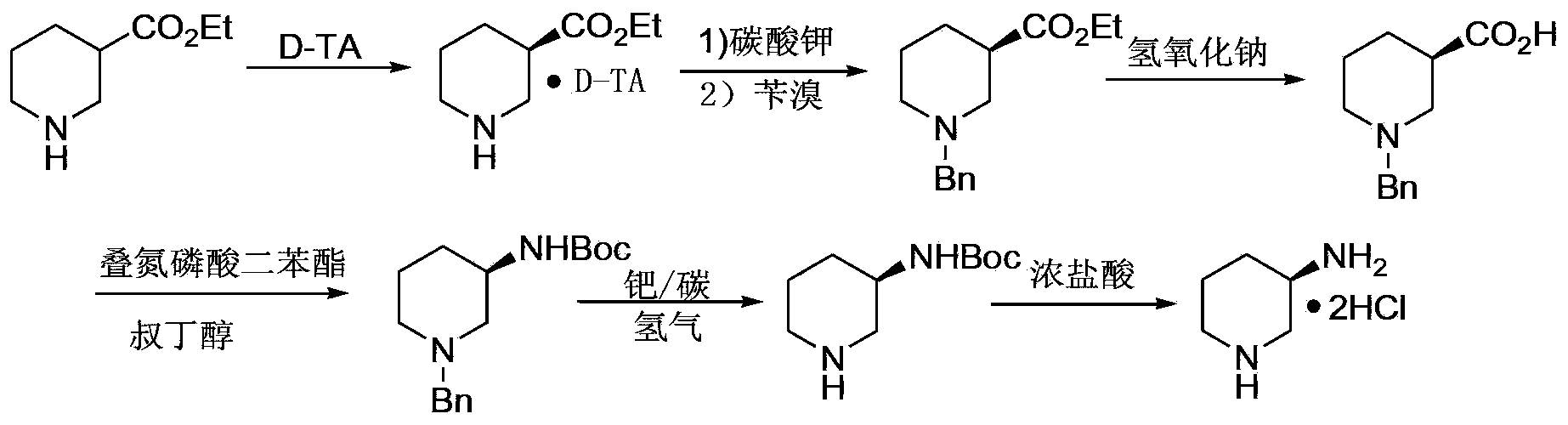
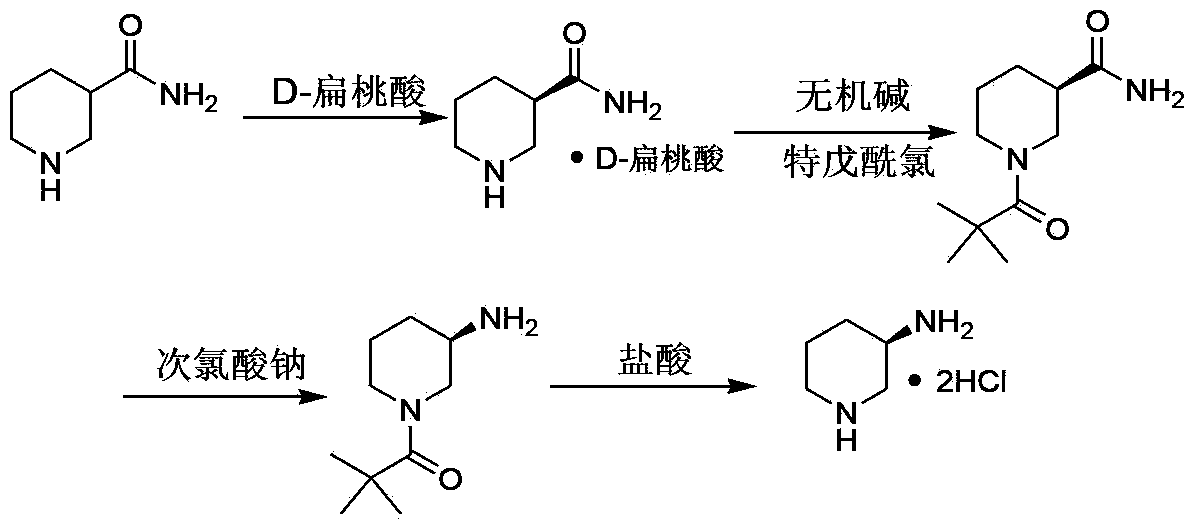
[0032] The preparation method of (R)-3-aminopiperidine hydrochloride, comprises the following steps:
[0033] a. React D-mandelic acid and racemic 3-piperidine amide in an organic solvent at 30-80°C for 5-12 hours to prepare D-mandelic acid and (R)-3-piperidine amide Precipitate the organic salt, filter to obtain the D-mandelic acid organic salt of (R)-3-piperidine amide;
[0034] b. Adjust the pH of D-mandelic acid and (R)-3-piperidine amide organic salt in a mixed solution of alcohol and water to 10-11 with inorganic agents, and then react with pivaloyl chloride at 0-30°C. React for 5 to 12 hours. After the reaction, extract with an organic solvent, dry, and concentrate to obtain (R)-N-pivaloyl-3-piperidine amide;
[0035] c. React (R)-N-pivaloyl-3-piperidine amide in 5-15% sodium hypochlorite solution at 20-70°C for 5-12 hours. After the reaction, use an organic solvent After extraction, the organic layer was dried, and the product (R)-N-pivaloyl-3-aminopiperidine was dis...
Embodiment 1
[0047] a. Add 51.0 grams of D-mandelic acid and 29.0 grams of racemic 3-piperidine amide into a 500 ml reaction flask, then add 80 ml of methyl tert-butyl ether and 80 ml of isopropanol to the reaction flask , heated to 70°C, reacted for 6 hours, naturally cooled to 20°C at the end of the reaction, stirred at this temperature for 3 hours, a large amount of solids precipitated, filtered with suction, and dried under reduced pressure to obtain (R)-3-piperidine amide D-mandelic acid organic salt 26.7 grams, yield 42.0%.
[0048] B, the organic salt of the D-mandelic acid of 26.7 grams and (R)-3-piperidine amide, the sodium hydroxide of 10 grams join in the 500 milliliter reaction bottle, then add the ethanol of 90 milliliters and 60 milliliters in the reaction bottle Milliliters of water, after adding, stirred for 30 minutes, added 13.0 grams of pivaloyl chloride, reacted for 10 hours at 5°C, and after TLC followed the reaction, added 120 grams of water to the reaction solution t...
Embodiment 2
[0054] a, 18.0 grams of D-mandelic acid and 14.0 grams of racemic 3-piperidine amide are added to a 250 milliliter reaction flask, then 40 milliliters of ethyl acetate and 40 milliliters of n-propanol are added to the reaction flask, heated to 65°C, reacted for 4 hours, naturally cooled to 20°C after the reaction was completed, stirred at this temperature for 2 hours, a large amount of solids precipitated, filtered with suction, and dried under reduced pressure to obtain D of (R)-3-piperidine amide - 12.7 grams of mandelic acid organic salt, yield 41.5%.
[0055] B, the organic salt of the D-mandelic acid of 12.7 grams and (R)-3-piperidine amide, the sodium carbonate of 13 grams are joined in the reaction bottle of 250 milliliters, then the ethanol of 25 milliliters and 30 milliliters are added in the reaction bottle After the addition of water, after stirring for 20 minutes, add 7.0 grams of pivaloyl chloride and react at 10°C for 8 hours. After the reaction is completed by T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com