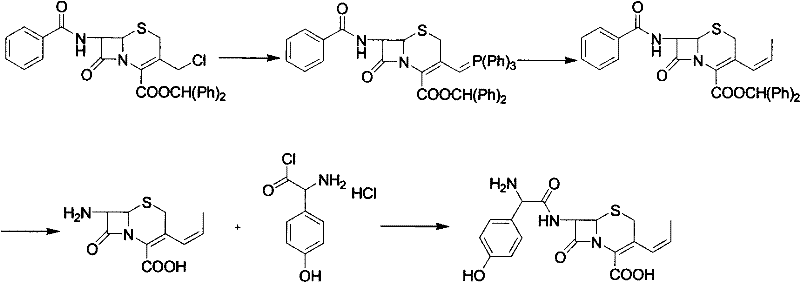
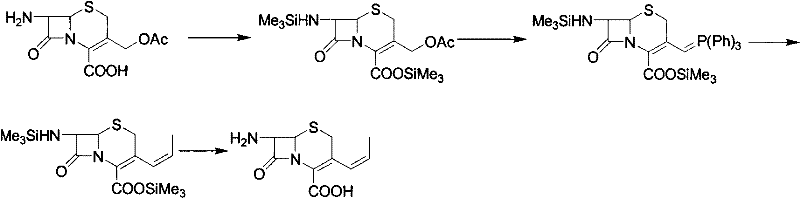
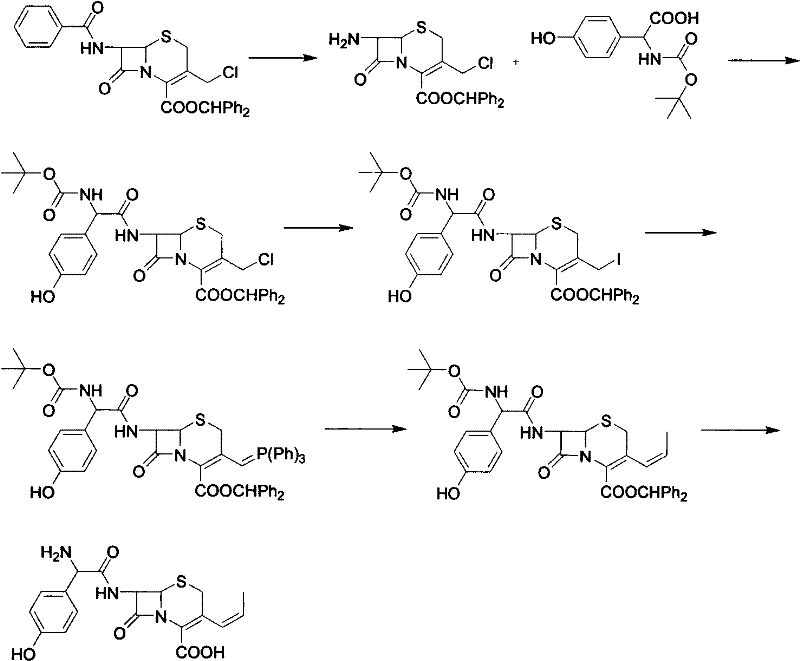
Preparation method of cefprozil
A technology of cefprozil and organic solvent, which is applied in the field of preparation of cefprozil, and can solve the problems of difficult acquisition of starting materials, complicated operation and long synthesis steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Under nitrogen protection, add 35 grams of 7-amino-3-propenylcephalosporanic acid and 200 milliliters of dichloromethane into a three-necked flask, cool down to -45~-55 ° C, add 18 grams of tetramethylguanidine, stir to dissolve, and control Warm to -20~-25°C, keep warm for later use. In another flask, add 100ml of dichloromethane, 100ml of N,N-dimethylformamide, 300ml of picolin, cool down to -50°C, add 55g of p-hydroxydane, and control the temperature at about -50°C Add 22 grams of pivaloyl chloride, after the addition is complete, keep the temperature at -20~-25°C for 1 hour, cool down to -70°C, add a dichloromethane solution of 7-amino-3-propenylcephalosporanic acid, and complete the addition. Raise the temperature to -20~-25°C, control the temperature and react for 2 hours, monitor 7-amino-3-propenyl cephalosporanic acid <<3% by HPLC, stop the reaction, add 90 ml of water and 37 g of hydrochloric acid, heat up to 5°C , adjust pH=1.5, stir for 30 minutes, stand to ...
Embodiment 2
[0020] Under the protection of nitrogen, add 32 grams of 7-amino-3-propenyl cephalosporanic acid and 200 milliliters of dichloroethane into the three-necked flask, cool down to -65~-75 ° C, add 18 grams of tetramethylguanidine, stir to dissolve, Control the temperature to -30~-35°C and keep warm for later use. In another flask, add 100ml of dichloroethane, 100ml of N,N-dimethylformamide, 300ml of picolin, cool down to -70°C, add 58g of p-Hydroxydan salt, and control the temperature at -70°C Add about 25 grams of pivaloyl chloride, after the addition, keep the temperature at -15~-20°C for 1 hour, cool down to -70°C, add the dichloroethane solution of 7-amino-3-propenylcephalosporanic acid, add Complete, heat up to -15~-20 ℃, temperature control reaction 2 hours, HPLC monitors to 7-amino-3-propenyl cephalosporanic acid <<3%, stop reaction, add 90 milliliters of water and 37 grams of hydrochloric acid, heat up to 0°C, adjust pH=2.8, stir for 30 minutes, stand to separate layers,...
Embodiment 3
[0022] Under nitrogen protection, add 33 grams of 7-amino-3-propenyl cephalosporanic acid and 200 milliliters of dichloroethane into the three-necked flask, cool down to -30~-35 °C, add 19 grams of tetramethylguanidine, stir to dissolve, Control the temperature to -5 ~ 0 ℃, keep warm for later use. In another flask, add 100 ml of dichloroethane, 100 ml of N,N-dimethylformamide, 280 ml of picolin, cool down to -30°C, add 59 g of p-hydroxydane, and control the temperature at -30°C Add about 24 grams of pivaloyl chloride, after the addition, keep the temperature at -5-0°C for 1 hour, cool down to -50°C, add the dichloroethane solution of 7-amino-3-propenylcephalosporanic acid, and finish the addition , heated up to 0-5°C, and reacted with temperature control for 2 hours. HPLC monitored that 7-amino-3-propenyl cephalosporanic acid <<3%, stopped the reaction, added 110 ml of water and 40 g of hydrochloric acid, and heated to 10°C. Adjust pH=2, stir for 30 minutes, stand to separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com