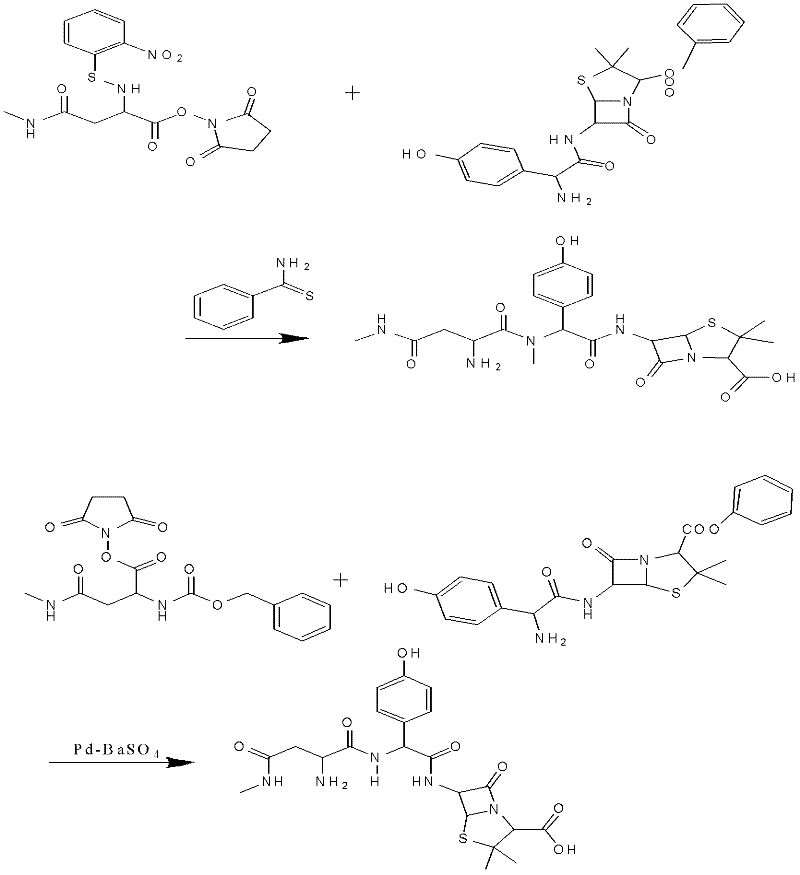
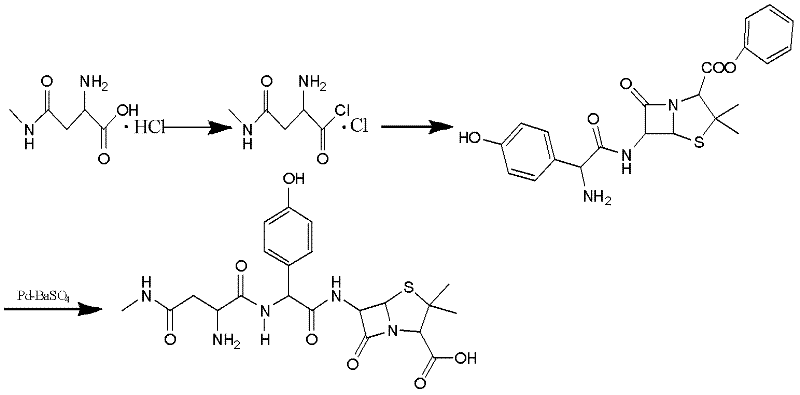
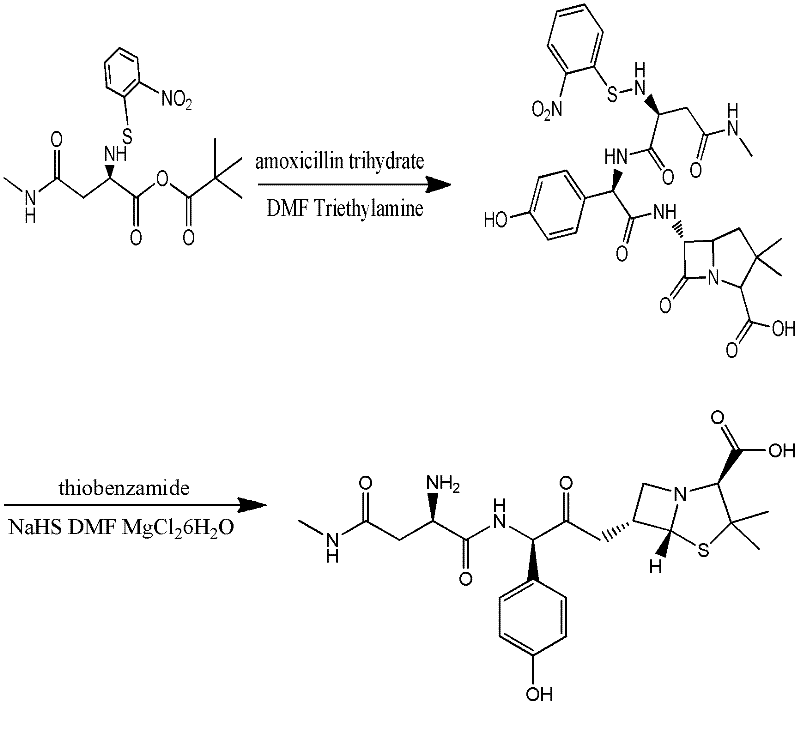
Preparation method for Aspoxicillin
A technology of apicillin and aspartic acid, which is applied in the field of the preparation of the first product of apicillin, can solve the problems of increasing the preparation cycle, unsafe industrial production, inconvenient deprotection and the like, and achieves easy operation, toxicity and environmental impact. The effect of low pressure, easy storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 apoxicillin
[0040] 1) synthesis of aspartate hydrochloride
[0041] Reaction formula:
[0042]
[0043] operate:
[0044] Add 100g (0.75mol) of D-Asp to 54ml (0.75mol) of thionyl chloride and 500ml of methanol liquid under stirring at a temperature of 0-10°C (thionyl chloride is added dropwise to methanol under stirring, and the exothermic phenomenon is obvious during this process ), the reaction solution was colorless and transparent, and after the addition, continued to stir at room temperature and reacted for 40 minutes before doing TLC analysis (TLC: n-butanol: water: acetic acid = 3: 1: 1, the product was yellow-brown on the top, and the raw material was yellow-brown on the bottom). purple), concentrated under reduced pressure at 40°C to dryness to obtain a white solid, and then recrystallized the obtained solid with 50ml of methanol and 200ml of ethyl acetate, and a large amount of white solid was precipitated, filtered with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com