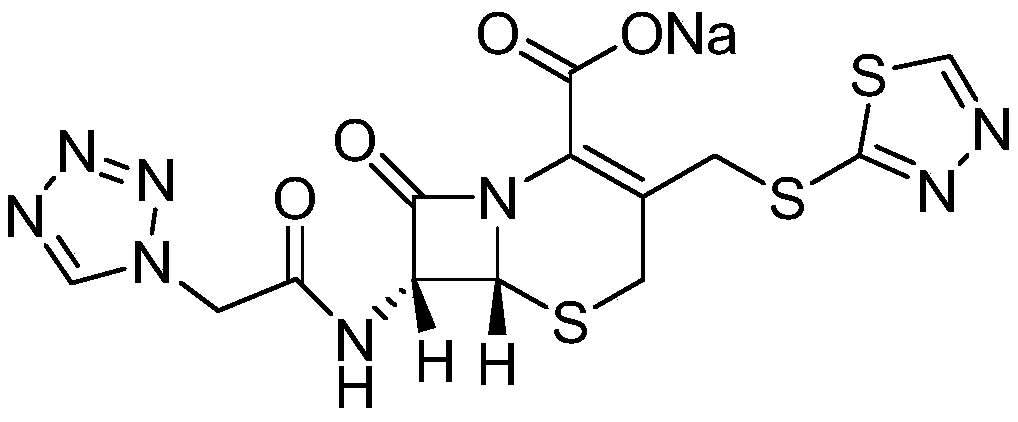
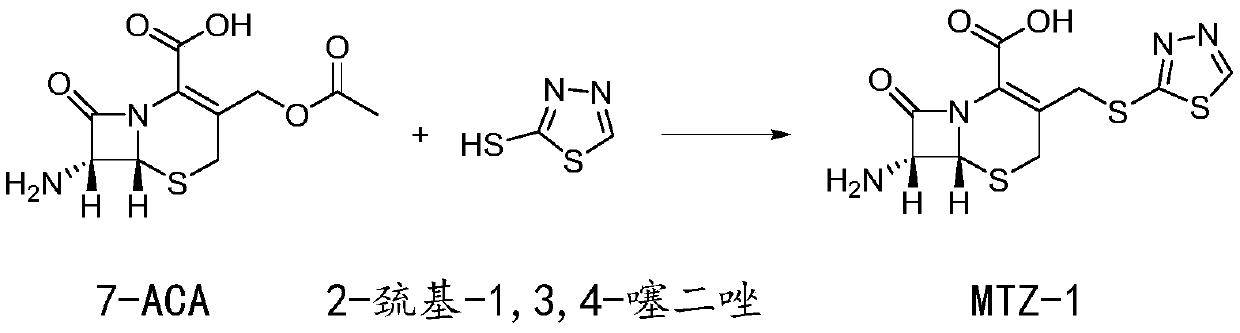
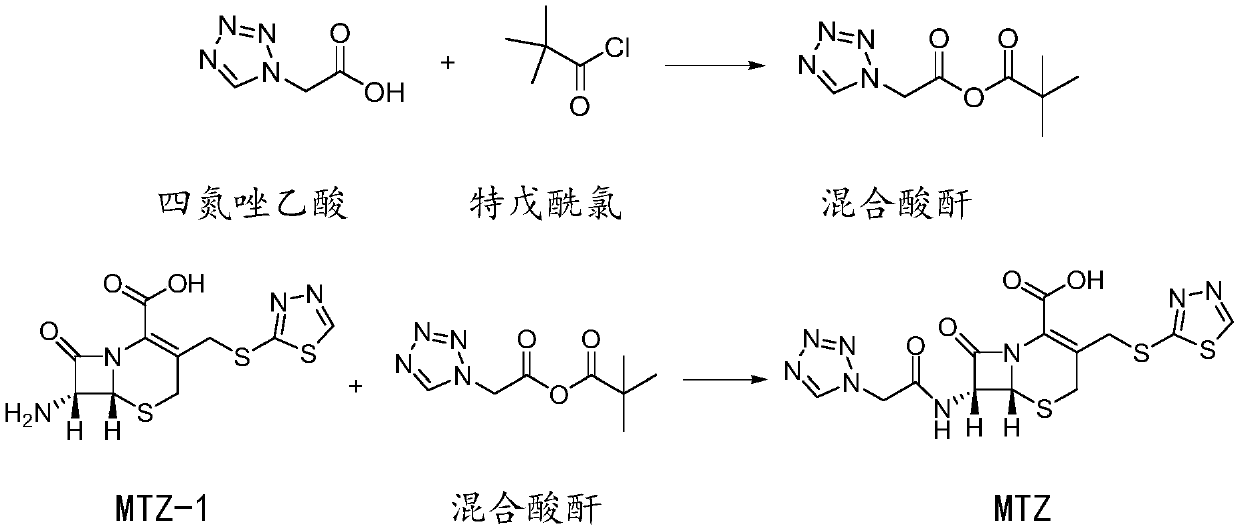
Preparation method of ceftezole acid
A technology of ceftezole acid and tetrazole acetic acid, which is applied in the field of preparation of ceftezole acid, can solve the problems of complex process flow, high production cost, cumbersome steps, etc., and achieve simple process flow, low cost and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation method of the ceftezole acid of the present embodiment, comprises the steps:
[0049] Step 1: Production of intermediate MTZ-1
[0050] Add 33.0kg of dimethyl carbonate, 5.0kg of anhydrous formic acid, 11.0kg of 7-ACA and 5.5kg of 2-mercapto-1,3,4-thiadiazole into the reaction kettle, cool down to 0°C while stirring; then add 33.0 kg of boron trifluoride dimethyl carbonate was controlled at a temperature of 10° C., and reacted for 50 minutes to obtain the MTZ-1 reaction solution.
[0051] Transfer the MTZ-1 reaction solution into water with 2.0 times the weight of the reaction solution, adjust the pH value to 1.0 with sodium carbonate solid, centrifuge at 600 rpm for 6 hours, and dry at 45°C for 7 hours with a boiling drying bed , to obtain 11.9kg intermediate MTZ-1.
[0052] Step 2: Production of MTZ
[0053] Add 100kg of dichloromethane and 5.5kg of tetrazoleacetic acid into the reaction kettle, cool to -10°C, add triethylamine dropwise under stirri...
Embodiment 2
[0057] The preparation method of the ceftezole acid of the present embodiment, comprises the steps:
[0058] Step 1: Production of intermediate MTZ-1
[0059] Add 44.0kg of dimethyl carbonate, 5.5kg of anhydrous formic acid, 11.0kg of 7-ACA and 4.9kg of 2-mercapto-1,3,4-thiadiazole into the reaction kettle, and cool down to 10°C while stirring; 37.5 kg of boron trifluoride acetonitrile, the temperature is controlled at 20°C, and after 60 minutes of reaction, the MTZ-1 reaction solution is obtained.
[0060] Transfer the MTZ-1 reaction solution into water with 2.5 times the weight of the reaction solution, adjust the pH value to 2.0 with ammonia water, centrifuge at 800 rpm for 5.5 hours, and dry at 50°C for 6.5 hours in a blast drying oven. 12.2 kg of intermediate MTZ-1 were obtained.
[0061] Step 2: Production of MTZ
[0062] Add 100kg of dichloromethane and 4.90kg of tetrazoleacetic acid into the reaction kettle, cool to -10°C, add triethylamine dropwise under stirring u...
Embodiment 3
[0066] The preparation method of the ceftezole acid of the present embodiment, comprises the steps:
[0067] Step 1: Production of intermediate MTZ-1
[0068] Add 50.0kg of dimethyl carbonate, 5.5kg of anhydrous formic acid, 11.0kg of 7-ACA and 7.0kg of 2-mercapto-1,3,4-thiadiazole into the reaction kettle, and cool down to 20°C while stirring; 50.0 kg of boron trifluoride diethyl ether was controlled at a temperature of 30° C., and reacted for 75 minutes to obtain a MTZ-1 reaction solution.
[0069] Transfer the MTZ-1 reaction solution into water with 3.0 times the weight of the reaction solution, adjust the pH value to 3.0 with triethylamine, centrifuge at 1000 rpm for 5 hours, and dry at 55°C for 6 Hours, 12.0kg intermediate MTZ-1 was obtained.
[0070] Step 2: Production of MTZ
[0071] Add 100kg of dichloromethane and 4.5kg of tetrazoleacetic acid into the reaction kettle, cool to -10°C, add triethylamine dropwise under stirring until the tetrazoleacetic acid is comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com