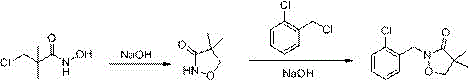Preparation method of clomazone
A technology of clomazone and hydroxyl, which is applied in the field of preparation of the herbicide clomazone, can solve the problems that are not suitable for industrial production, the rate of increase in yield is small, and the processing steps are cumbersome, and achieve good industrial application prospects. Effects with simple controls, easy recycling and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Step 1. Add 18.8g (0.26mol) hydroxylamine hydrochloride, 100ml distilled water, 2g ether catalyst into a 250ml four-neck flask, stir under ice salt bath (-5~0℃), add 30% NaOH solution dropwise to make the solution pH The value reaches 7.2. Then 6ml (0.2mol) of chloropivaloyl chloride was added dropwise, and 30% NaOH solution was added dropwise to keep the system pH 7.2±0.1. After the dropwise addition was completed within 1.5h, the mixture was stirred at room temperature for 3h. After the completion of the reaction, after filtering and drying, the white solid obtained is 3-chloro-N-hydroxy-2,2-dimethylpropionamide. The content is 99.0% (HPLC), and the yield is 95.3%.
[0024] Step two
[0025] Add 15.2g of 3-chloro-N-hydroxy-2,2-dimethylpropionamide (prepared in the first step) to a 250ml reaction flask equipped with a pH meter, a constant pressure dropping funnel, a thermometer, and a mechanical stirring device. 55ml distilled water, stirring vigorously at 45°C. Add 30%...
example 2
[0028] Step 1. Add 18.8g (0.26mol) hydroxylamine hydrochloride, 100ml distilled water, 2g ether catalyst into a 250ml four-neck flask, stir under ice salt bath (-5~0℃), add 50% NaOH solution dropwise to make the solution pH The value reaches 7.2. Then 6ml (0.2mol) of chloropivaloyl chloride was added dropwise, and 50% NaOH solution was added dropwise to keep the system pH 7.2±0.1. After the dropwise addition was completed within 1.5h, the mixture was stirred at room temperature for 3h. After the completion of the reaction, after filtering and drying, the white solid obtained is 3-chloro-N-hydroxy-2,2-dimethylpropionamide. The content is 99.0% (HPLC), and the yield is 93.3%.
[0029] Step two
[0030] Add 15.2g of 3-chloro-N-hydroxy-2,2-dimethylpropionamide (prepared in the first step) to a 250ml reaction flask equipped with a pH meter, a constant pressure dropping funnel, a thermometer, and a mechanical stirring device. 55ml distilled water, stirring vigorously at 45°C. Add 30%...
example 3
[0033] Step 1: Add 18.8g (0.26mol) hydroxylamine hydrochloride, 100ml distilled water, 2g ether catalyst into a 250ml four-neck flask, stir under ice salt bath (-5~0℃), add 30% KOH solution dropwise to make the solution pH The value reaches 7.2. Then 6ml (0.2mol) of chloropivaloyl chloride was added dropwise, while 30% KOH solution was added dropwise to maintain the pH of the system at 7.2±0.1. After the dropwise addition was completed within 1.5 hours, the mixture was stirred at room temperature for 3 hours. After the completion of the reaction, after filtering and drying, the white solid obtained is 3-chloro-N-hydroxy-2,2-dimethylpropionamide. The content is 99.0% (HPLC), and the yield is 94.3%.
[0034] Step two
[0035] Add 15.2g of 3-chloro-N-hydroxy-2,2-dimethylpropionamide (prepared in the first step) to a 250ml reaction flask equipped with a pH meter, a constant pressure dropping funnel, a thermometer, and a mechanical stirring device. 55ml distilled water, stirring vigo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com