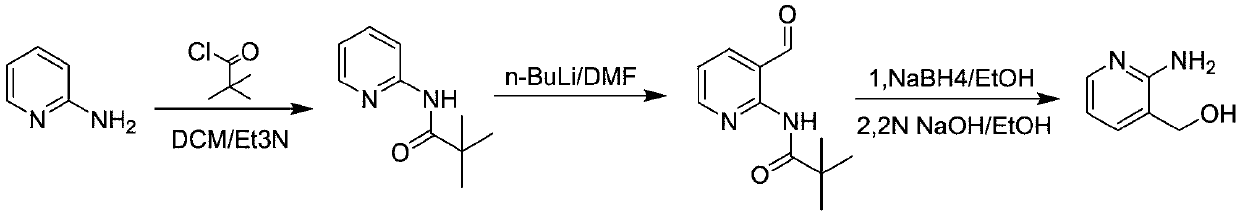
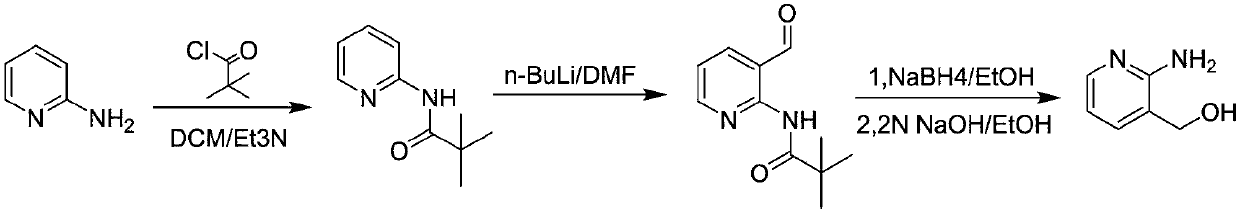
Method for preparing 2-amino-3-hydroxymethylpyridine
A technology of hydroxymethylpyridine and aminopyridine, which is applied in the field of preparation of 2-amino-3-hydroxymethylpyridine, can solve the problems of expensive excipients, high reaction temperature, large production sewage, etc., and achieve low raw material cost , high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of 2-amino-3-hydroxymethylpyridine:
[0034] S1. At room temperature, add dichloromethane (900g) into a 2L three-necked flask, add 2-aminopyridine (135g, 1.0eq) and triethylamine (174g, 1.2eq) in sequence, cool to 10-20°C, Pivaloyl chloride (189g, 1.1eq) was added dropwise. After the dropwise addition, the system was warmed up to 20-25°C and reacted for 2-3h;
[0035] After the reaction was completed, water was added to the reaction system, and after stirring for 0.5 h, the organic phase was separated, the aqueous phase was extracted with dichloromethane (500 g), and the organic phase was concentrated to dryness. At 0°C, petroleum ether (800 g) was directly added, stirred for 2 h, and suction filtered to obtain 2-pivalamidopyridine with a yield of 83%. NMR detection: 1H-NMR (400MHz, CDCl 3 ):δ8.23(2H,m,J=8Hz),8.03(1H,s),7.65(1H,t,J=4Hz),7.00(1H,t,J=4Hz),1.30(9H,s) .
[0036] S2. Under the protection of nitrogen, the above-mentioned 2-piv...
Embodiment 2
[0041] A kind of preparation method of 2-amino-3-hydroxymethylpyridine:
[0042] S1. At room temperature, add dichloromethane (945g) into a 2L three-necked flask, add 2-aminopyridine (135g, 1.0eq) and triethylamine (188g, 1.3eq) in sequence, cool to 10-20°C, Pivaloyl chloride (198g, 1.15eq) was added dropwise. After the dropwise addition, the system was warmed to 20-25°C and reacted for 2-3h;
[0043] After the reaction was completed, water was added to the reaction system, and after stirring for 0.5 h, the organic phase was separated, the aqueous phase was extracted with dichloromethane (500 g), and the organic phase was concentrated to dryness. At 0°C, petroleum ether (800 g) was directly added, stirred for 2 h, and suction filtered to obtain 2-pivalamidopyridine with a yield of 84%. NMR detection: 1H-NMR (400MHz, CDCl 3 ):δ8.23(2H,m,J=8Hz),8.03(1H,s),7.65(1H,t,J=4Hz),7.00(1H,t,J=4Hz),1.30(9H,s) .
[0044] S2. Under the protection of nitrogen, the above-mentioned 2-pivalam...
Embodiment 3
[0049] A kind of preparation method of 2-amino-3-hydroxymethylpyridine:
[0050] S1. At room temperature, add dichloromethane (900g) into a 2L three-necked flask, add 2-aminopyridine (135g, 1.0eq) and triethylamine (174g, 1.2eq) in sequence, cool down to 15°C, add dropwise Pivaloyl chloride (189g, 1.1eq), after the dropwise addition, the system was warmed up to 20-25°C, and reacted for 2-3h;
[0051] After the reaction was completed, water was added to the reaction system, and after stirring for 0.5 h, the organic phase was separated, the aqueous phase was extracted with dichloromethane (500 g), and the organic phase was concentrated to dryness. At 0°C, petroleum ether (800 g) was directly added, stirred for 2 h, and suction filtered to obtain 2-pivalamidopyridine with a yield of 80.8%. NMR detection: 1H-NMR (400MHz, CDCl 3 ):δ8.23(2H,m,J=8Hz),8.03(1H,s),7.65(1H,t,J=4Hz),7.00(1H,t,J=4Hz),1.30(9H,s) .
[0052] S2. Under the protection of nitrogen, the above-mentioned 2-piva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com