Patents
Literature
45results about How to "No harsh reaction conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
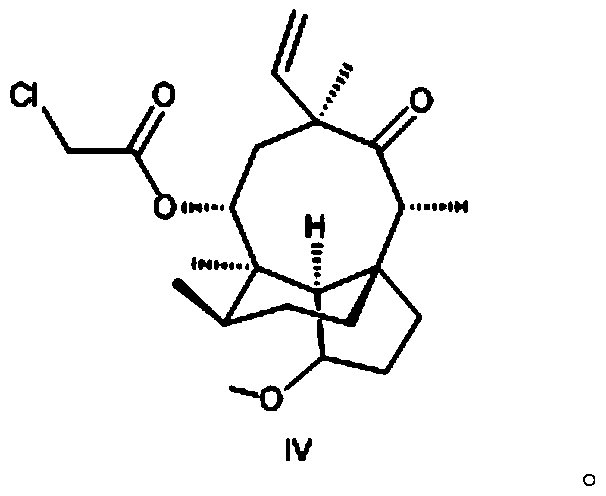
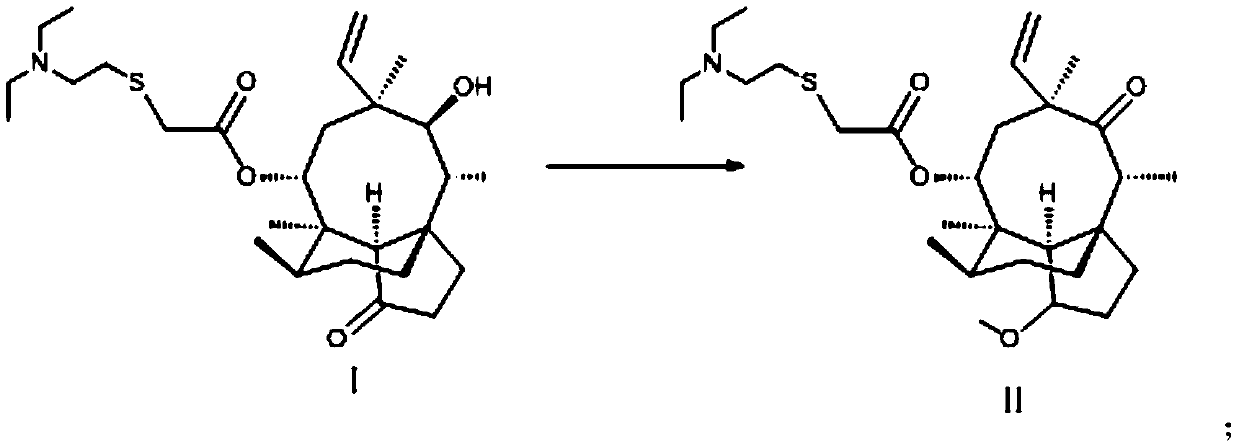
Preparation method of methyl octabromo-ether
InactiveCN109336746AReduce broken linksReducing the Possibility of Methyl BromideEther preparation by ester reactionsTetrabromobisphenol ASulfite salt
The invention discloses a preparation method of methyl octabromo-ether and belongs to the technical field of fire retardant methyl octabromo-ether production. The preparation method comprises the following steps: dissolving tetrabromobisphenol A in water, regulating a pH (potential of hydrogen) value with sodium hydroxide, performing heat preservation for the first time, dropwise adding a methallyl chloride organic solution, adding a phase transfer catalyst, after dropwise adding, performing heat preservation for the second time, then cooling to a room temperature, performing lamination, taking an organic layer, dissolving a brominating agent in an organic solvent to form a brominating agent solution, adding the organic layer into a catalyst, dropwise adding the brominating agent solutionfor brominating reaction, performing heat preservation for the third time at 25-55 DEG C, adding a sodium sulfite aqueous solution for reaction, washing with water after the reaction, standing for lamination, taking a lower organic layer, adding into an emolsifier aqueous solution, performing distillation to remove the organic solvent, performing suction filtration to form a material, and drying the material to prepare a product, wherein a mole ratio of the water to tetrabromobisphenol A is (2-8):1; and a range of the pH value is 8-10.
Owner:WEIFANG RIXING CHEM
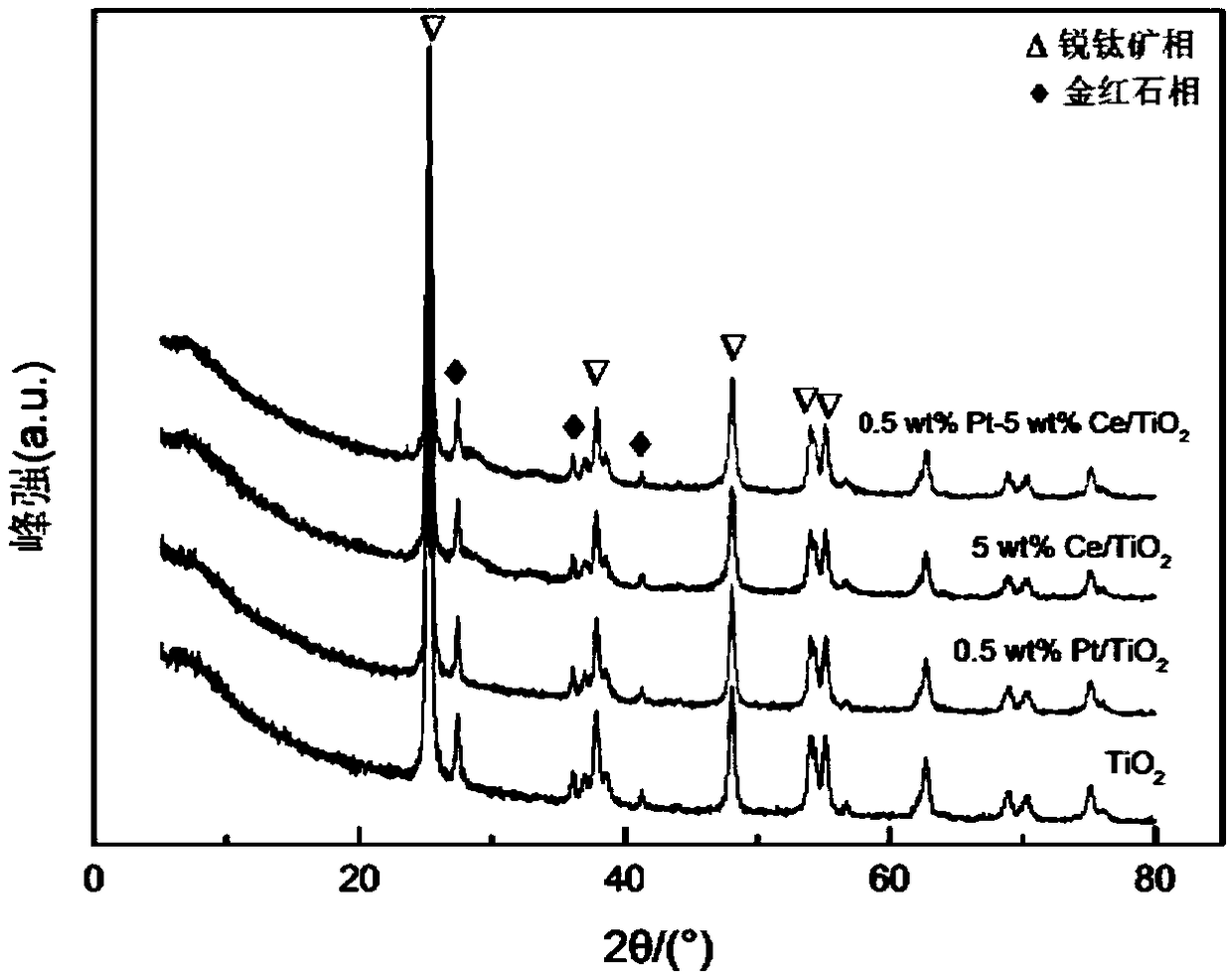
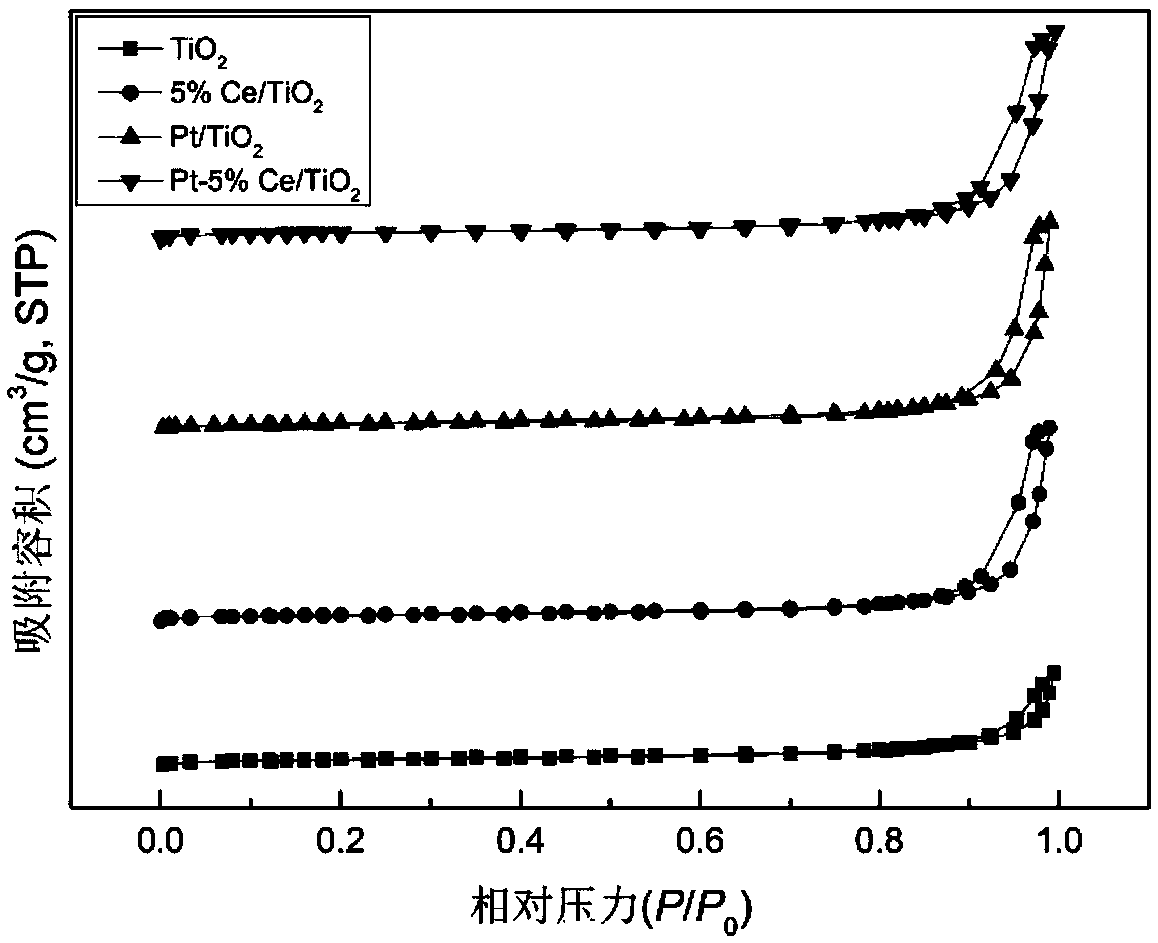
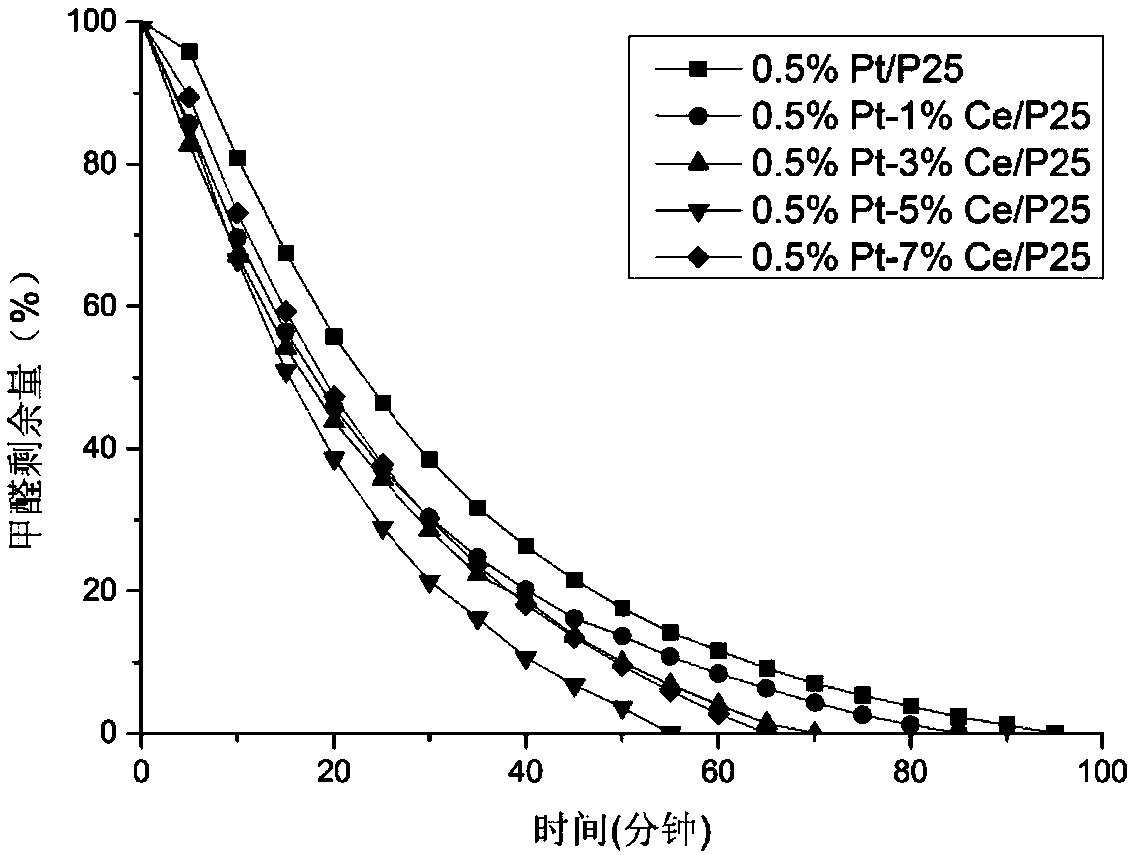
Alloy catalyst for degrading formaldehyde, and preparation method and application of alloy catalyst
InactiveCN108906043AImprove adsorption capacitySimple methodGas treatmentDispersed particle separationDispersityOxygen vacancy
The invention provides a preparation method of an alloy catalyst for degrading formaldehyde. The method comprises the following steps: (1) adding a TiO2 nano material and a cerium compound into water,carrying out impregnation and stirring to disperse cerium onto the TiO2 nano material, carrying out drying, grinding and roasting to obtain a Ce / TiO2 solid material; (2) adding the Ce / TiO2 solid material into water, carrying out stirring until the Ce / TiO2 solid material is completely dispersed, adding a platinum compound, and carrying out impregnation and stirring to disperse platinum onto the Ce / TiO2 solid material; (3) adding an alkali solution as a stabilizer, uniformly carrying out stirring and mixing, then adding a reducing agent, and carrying out a reduction reaction; and (4) separatingthe solid obtained in the step (3), carrying out water washing, alcohol washing, drying and cooling, and then carrying out grinding to obtain the Pt-Ce / TiO2 catalyst. The method is simple and feasible, and no harsh reaction conditions are required. According to the prepared Pt-Ce / TiO2 catalyst, the Pt nano particles are stabilized through the addition of the cerium according to the method, so that the dispersity is improved, the nano particle size is reduced, abundant oxygen vacancies are provided, and formaldehyde can be effectively decomposed.
Owner:GUANGZHOU UNIVERSITY
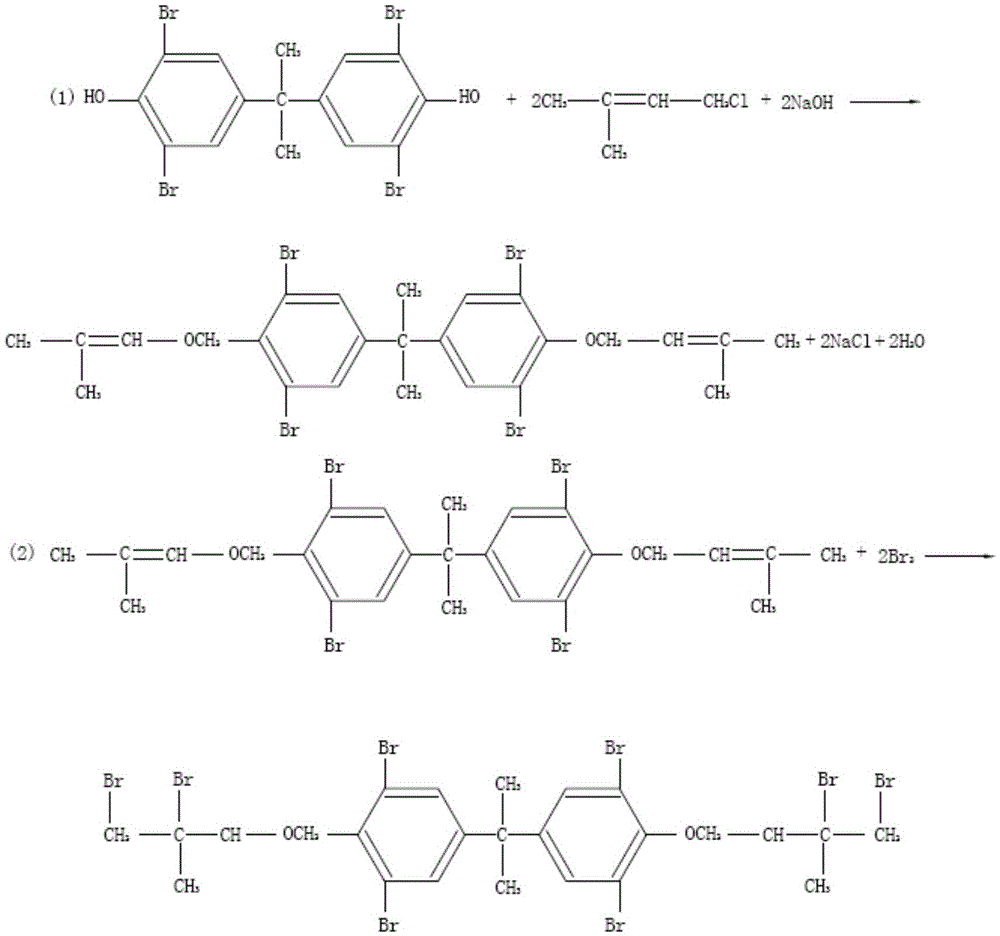
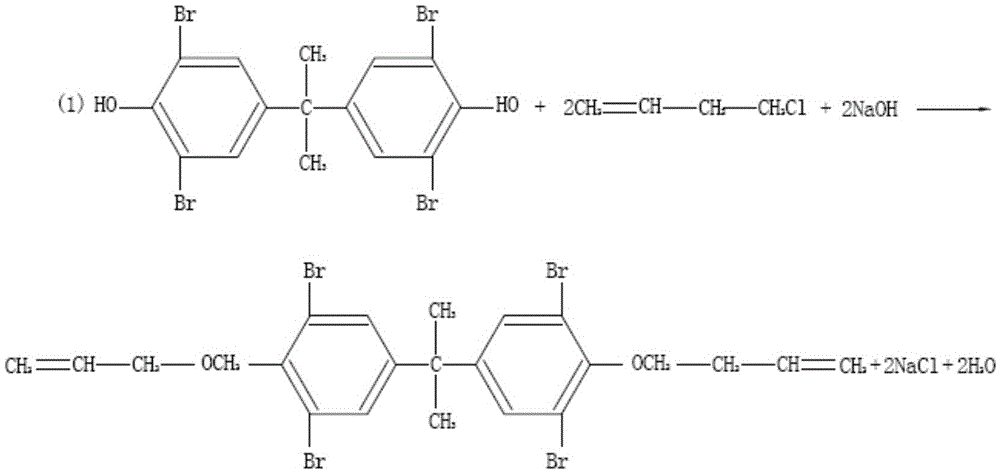
Synthesizing method of tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether
ActiveCN103193605ASimple methodReduce production energy consumptionOrganic chemistryOrganic compound preparationTetrabromobisphenol AReaction temperature
The invention discloses a synthesizing method of a tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether. The method comprises the following steps: adding tetrabromobisphenol and an alkali into water; stirring until completely dissolving; then carrying out the primary heat preservation; regulating the pH value; adding a dispersing agent; dropwise adding methylallyl chloride and carrying out the secondary heat preservation; after carrying out the primary aftertreatment, obtaining an intermediate product; adding the intermediate product into an organic solvent; stirring until the intermediate product is completely dissolved, and then dropwise adding the bromine; refluxing and carrying out the tertiary heat preservation; and after finishing the tertiary heat preservation, obtaining a product by carrying out the secondary aftertreatment. The method is simple; the adopted solvent can be recycled and reused; the temperature of the bromination reaction is low; the reaction condition is moderate and is easy to control; the energy consumption during the production is low; and the strict reaction conditions are not required by all steps, so that the method is favorable for industrial production. The tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether as the product is high in yield, good in quality, good in heat stability and high in content, thereby lightening the environmental-protection stress. In addition, the tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether is high in purity, low in impurity content and free of generating 'three wastes' and environmental pollution.
Owner:SHANDONG RUNKE CHEM
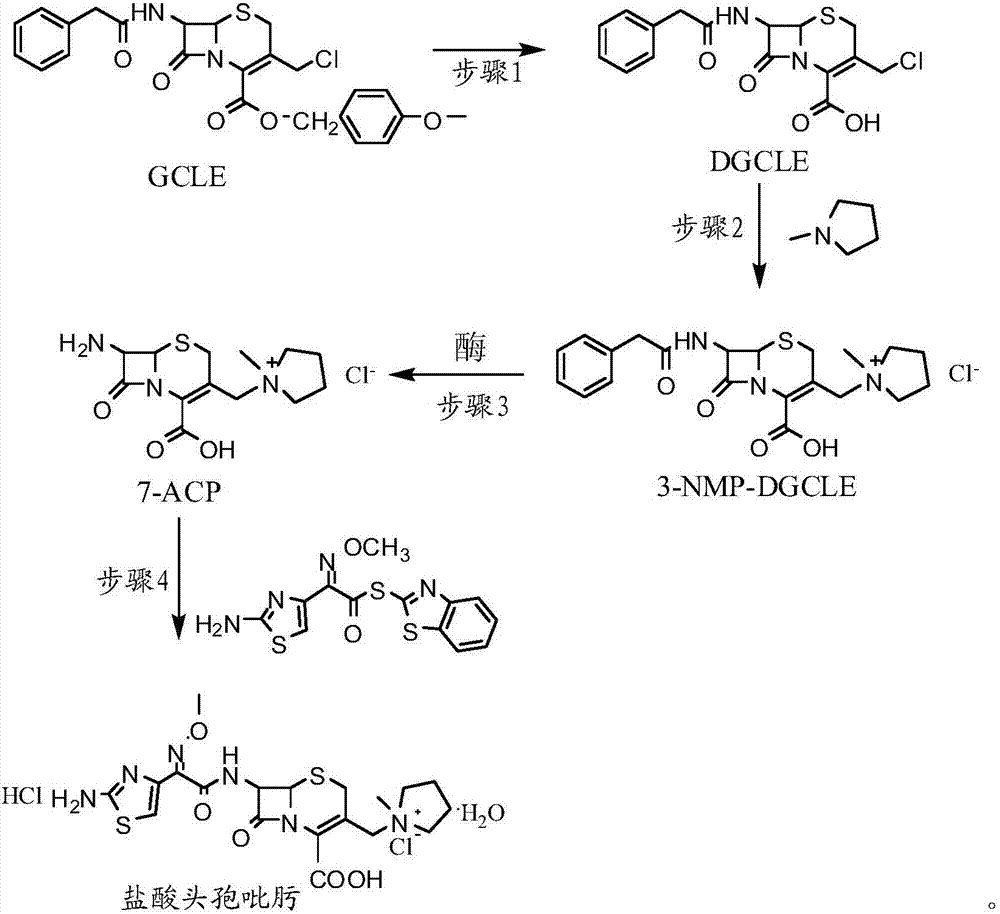
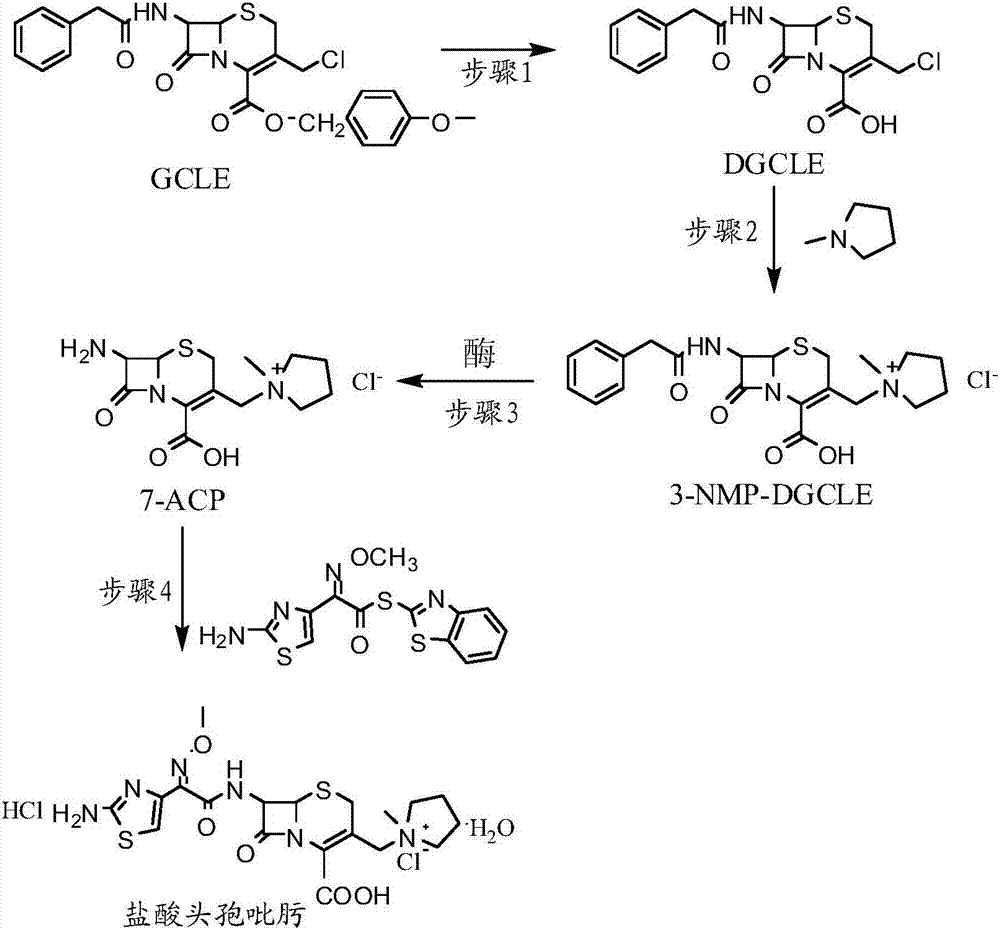
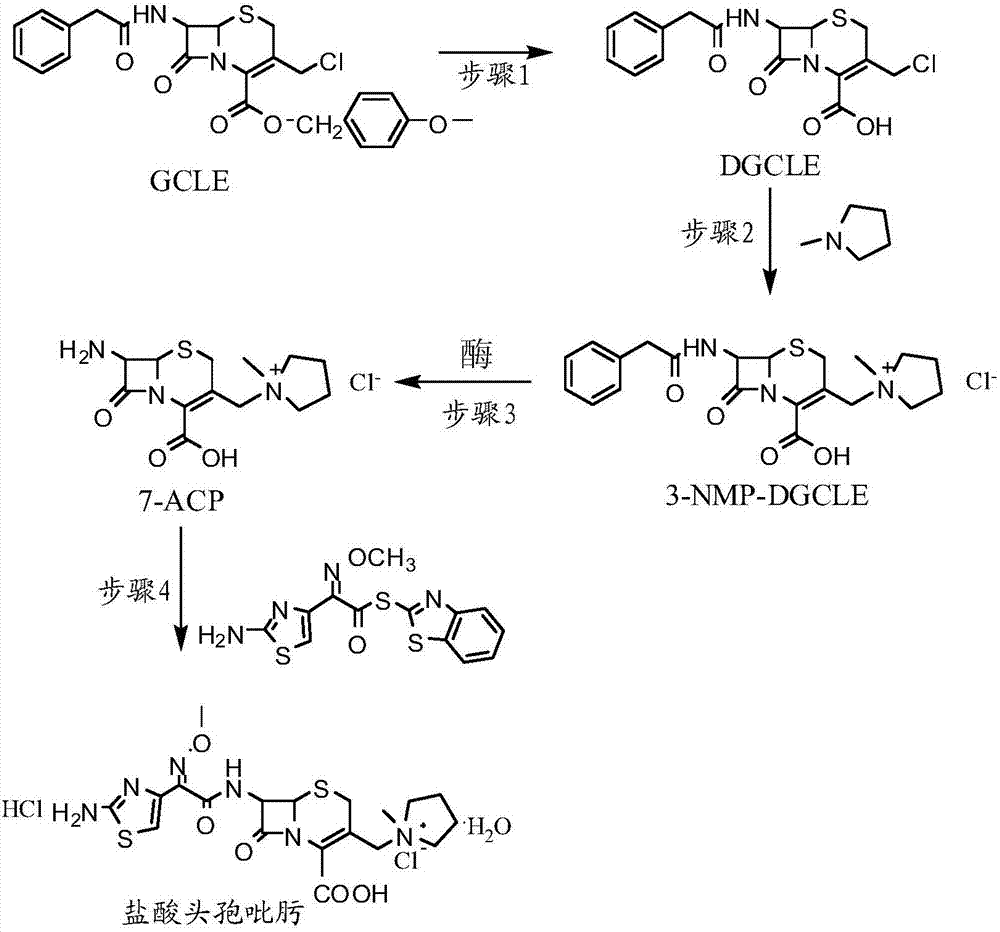
Synthesis method of cefepime hydrochloride
ActiveCN107201391AReduce usageLow costOrganic chemistryFermentationCefepime hydrochlorideSynthesis methods
The invention provides a synthesis method of cefepime hydrochloride. The synthesis method comprises the following steps: taking GCLE (7-phenylacetamide-3-chloromethyl-3-cepham-4-carboxylic acid p-methyl-oxybenzyl ester) as a raw material; cutting a 4-site protecting group (p-methoxybenzyl) and enabling the 4-site protecting group to react with N-methylpyrrolidine (NMP); then cutting a 7-site protecting group through an enzyme method to obtain an immediate 7-amino-3-(1-methylpyrrolidine)methyl)-3-cepham-4-carboxylic acid hydrochloride (7-ACP); taking cheap and available methoxyiminoacetic acid mercaptobenzothiazole active ester (AE-active ester) and the 7-ACP to be subjected to 7-site acylation reaction, so as to finally prepare the cefepime hydrochloride. A route provided by the invention can be used for obtaining the high-yield and high-quality cefepime hydrochloride without a delta2 isomer. The synthesis method provided by the invention has the advantages of simple process, no harsh reaction conditions and the like and is very suitable for industrial production.
Owner:吉林省爱诺德生物工程有限公司
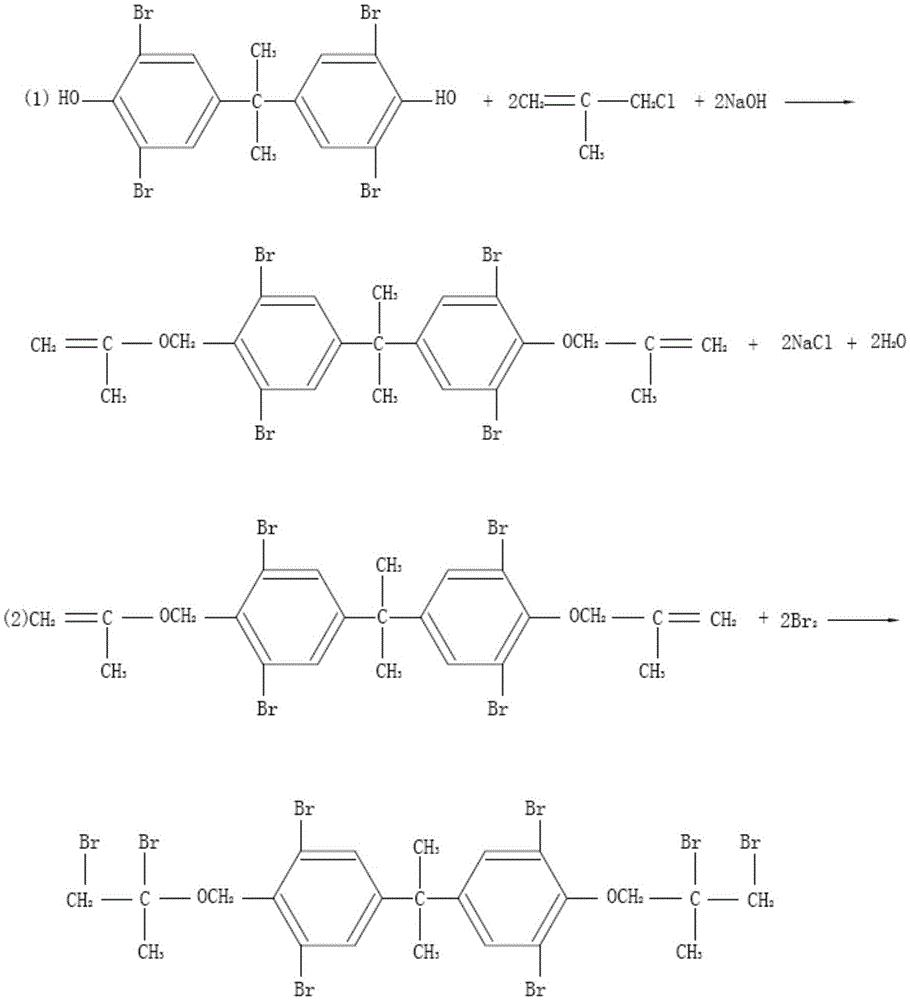
Preparation method of tetrabromobisphenol A bis(dibromoalkane)ether series compounds
InactiveCN105646163AReduce the production of by-productsLow reaction temperatureOrganic compound preparationEther preparation by ester reactionsBromineEther
The invention belongs to the technical field of compound preparation, and particularly relates to a preparation method of tetrabromobisphenol A bis(dibromoalkane)ether series compounds. The preparation method includes the following steps that tetrabromobisphenol A, methyl alcohol and water are mixed evenly, alkali is added and stirred to be completely dissolved, chloroalkene is dripped, and an intermediate is obtained; the intermediate is dissolved in an organic solvent, a catalyst is added and stirred to be completely dissolved, bromine is dripped, heat-preserved reflux is carried out, standing is carried out for layering after a reaction is finished, an organic layer is taken, elutriation crystallization, centrifugation and drying are carried out, and the tetrabromobisphenol A bis(dibromoalkane)ether series compounds are obtained. According to the method, the catalyst is added in the bromination process and can change the polarity of the organic solvent, so that by-products generated in the reaction process are reduced, and the content of the end product is increased by about 10%; the bromination reaction temperature is low, the reaction time is short, and reaction conditions are mild and easy to control; besides, production energy consumption is low, no rigorous reaction condition is needed in any step, the product yield is high, product quality is good, and the flame-retardant effect is good.
Owner:WEIFANG YUKAI CHEM
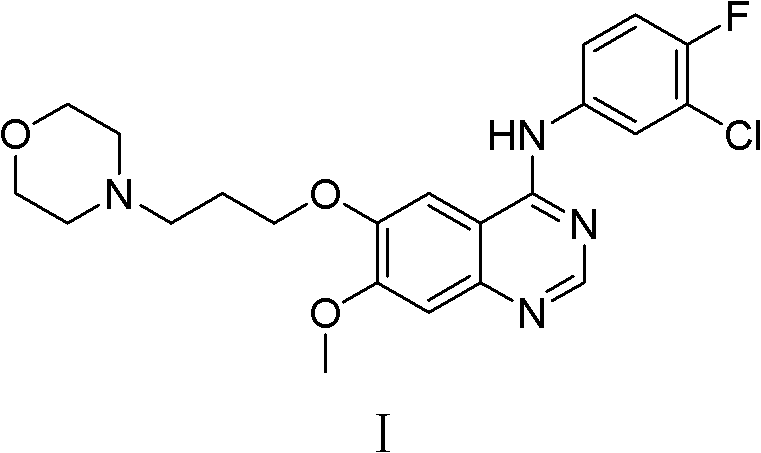
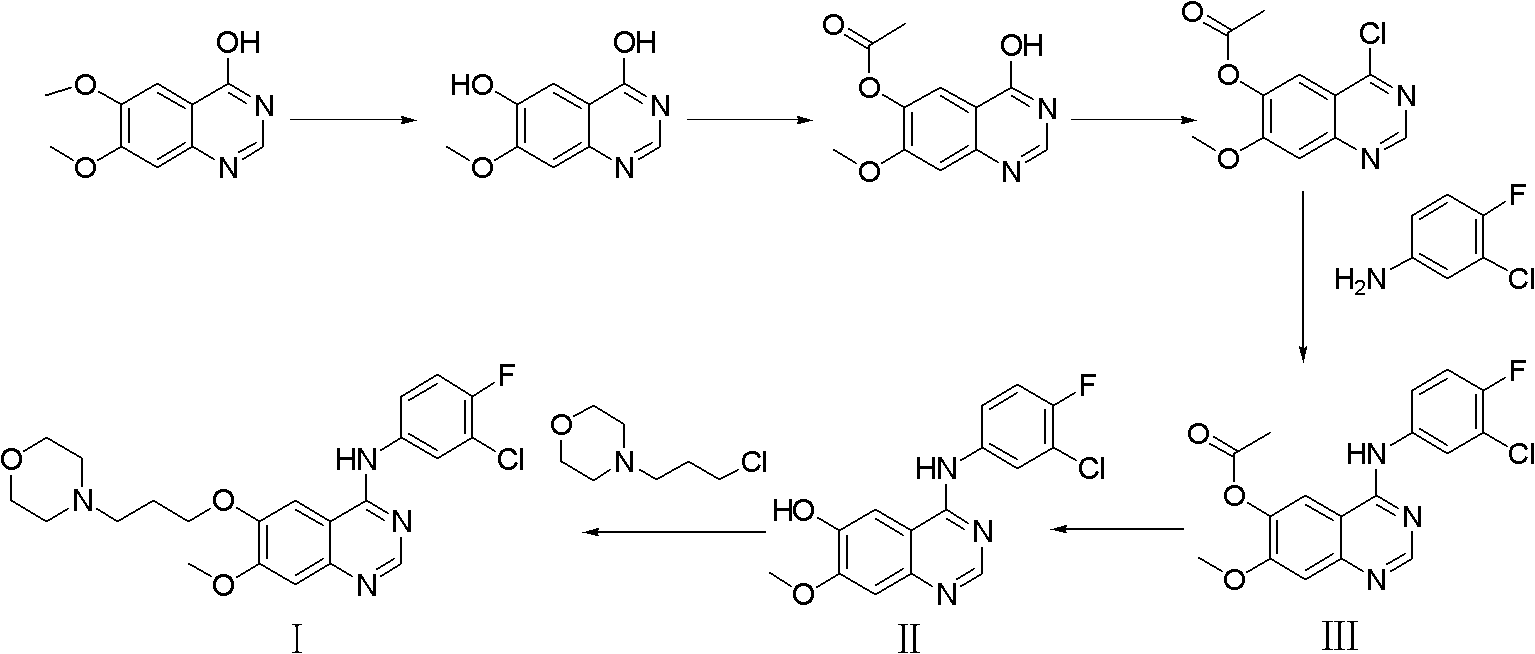
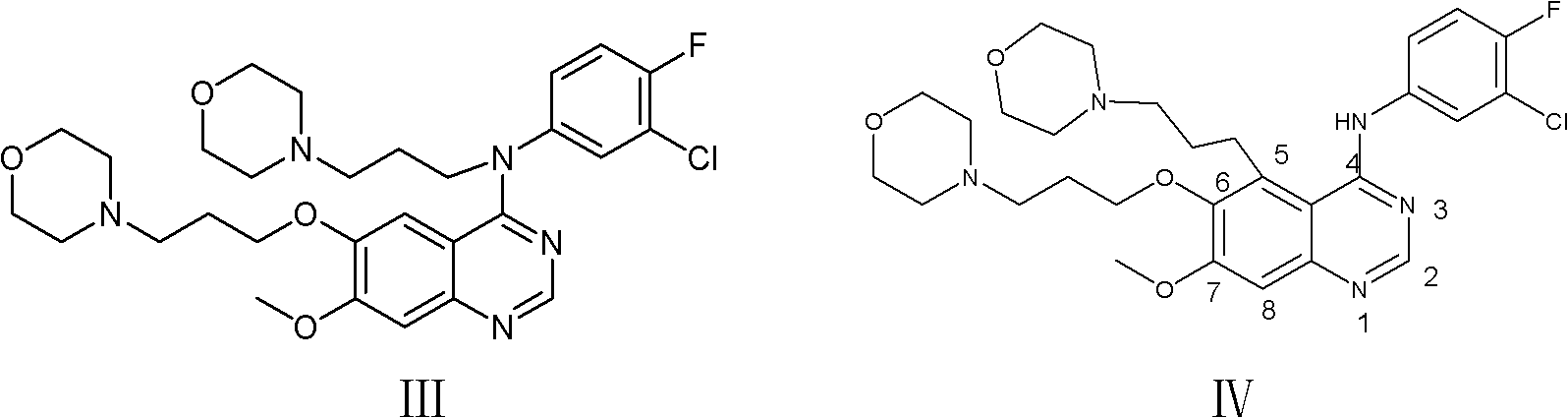
Preparation method of high-purity gefitinib
ActiveCN103012290ALong response time to resolveHigh yieldOrganic chemistryDimethyl formamideSODIUM SULFATE ANHYDROUS
The invention provides a preparation method of high-purity gefitinib. The preparation method includes the steps of adding 4-(3-chlorine-4-fluoroaniline)-7-methoxy-quinazoline-6-alcoholic compound II into N, N-dimethylformamide, then adding potassium carbonate with particle size d (0.5) less than or equal to 50microns, anhydrous sodium sulfate or anhydrous magnesium sulfate, and N-(3-chloropropyl) morpholine, heating to perform reaction, conduct aftertreatment to obtain the gefitini through postprocessing. According to the preparation method, the problems that the reaction time is long and more impurities exist during large-scale production are solved by controlling particle size of the potassium carbonate and adding the anhydrous sodium sulfate or the anhydrous magnesium sulfate; the preparation method has the advantages of simplicity in operation, easiness in refining, high yield and high product purity; and high-purity gefitinib is suitable for industrial production.
Owner:QILU PHARMA +1
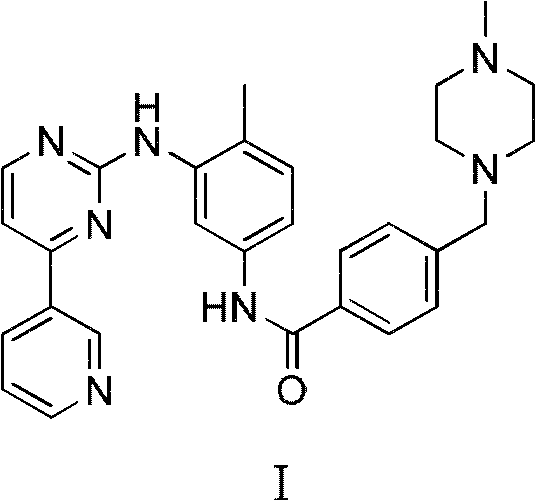
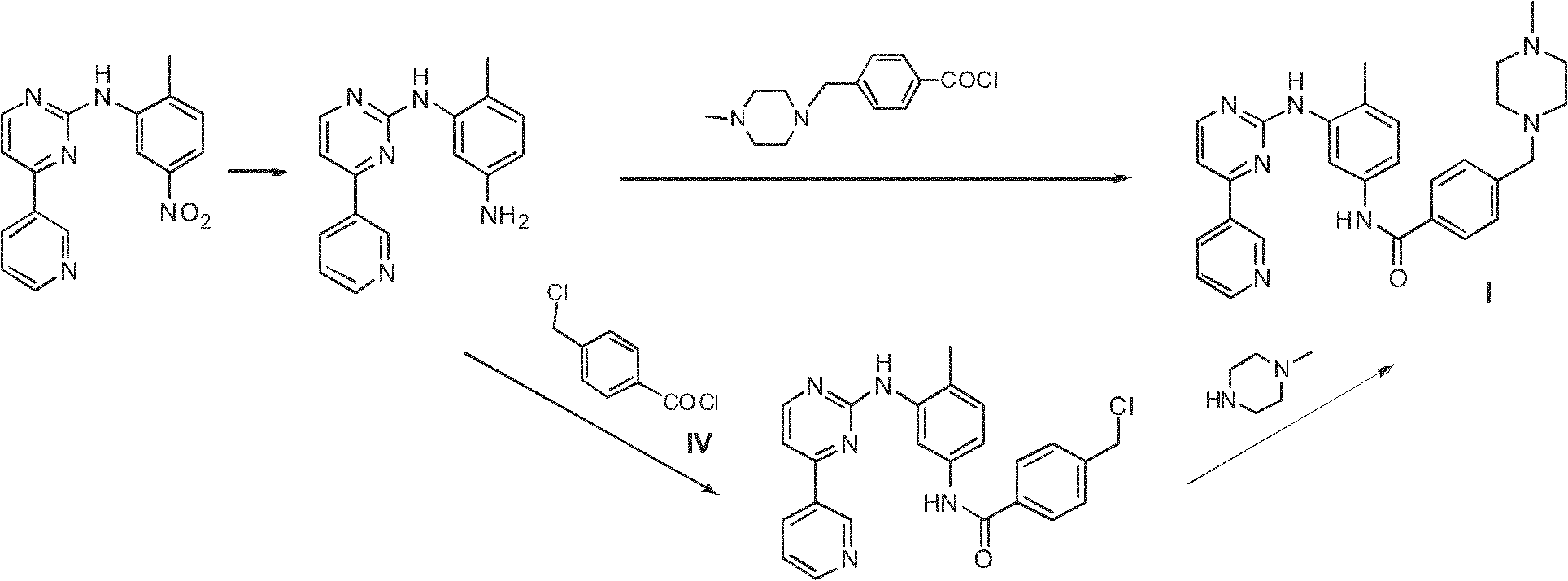
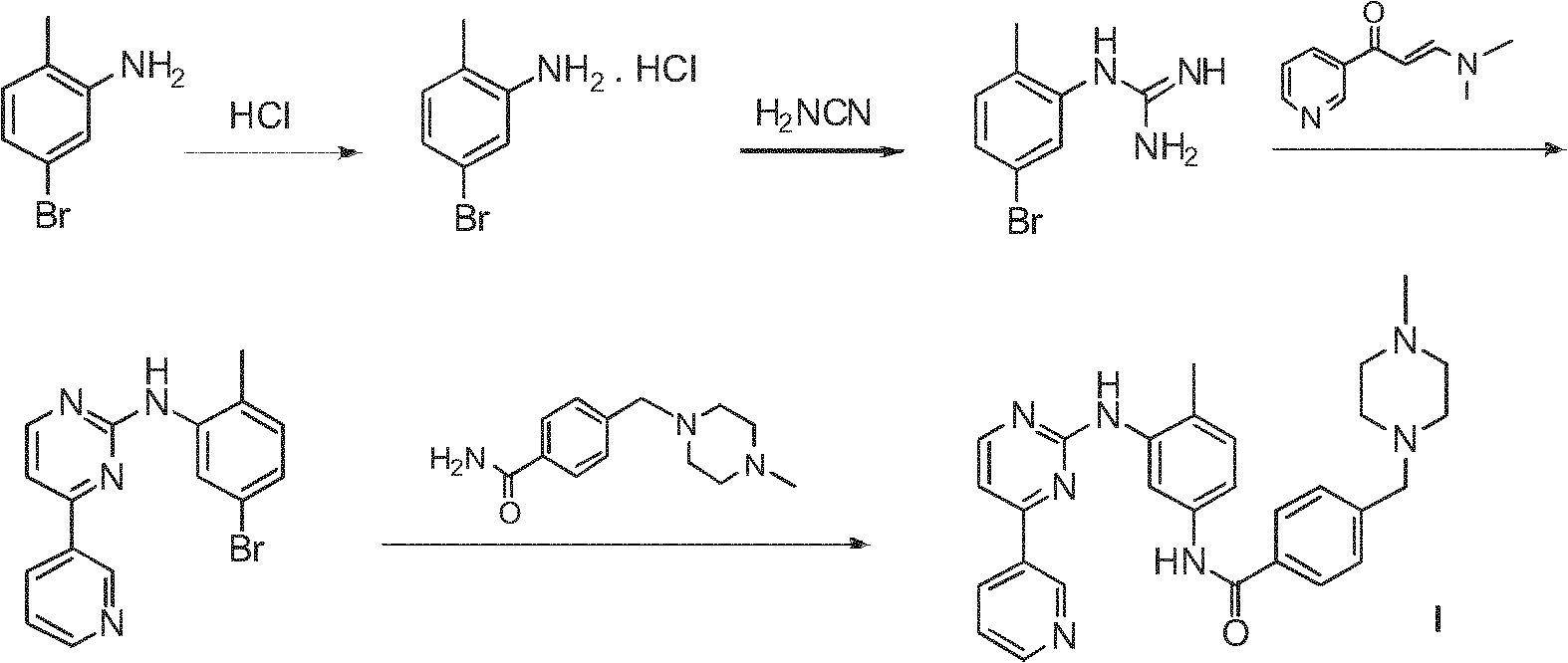
Method for preparing anti-tumor medicine imatinib
InactiveCN102234263AEconomicalMeet the requirementsOrganic chemistryAbnormal tissue growthBenzoyl chloride
The invention relates to a method for preparing an anti-tumor medicine imatinib, which comprises the following steps of: performing amidation-condensation two-step 'one pot' reaction on 4-methyl-3-bromaniline (V), 4-(chloromethyl) benzoyl chloride (IV) and N-methyl piperazine to directly obtain a key intermediate N-(4-methyl-3-bromophenyl)-4-(4-methyl piperazin-1-methyl)-benzamide (III); and performing nucleophilic substitution on the N-(4-methyl-3-bromophenyl)-4-(4-methyl piperazin-1-methyl)-benzamide (III) and 4-(3-Pyridinyl)-2-aminopyrimidine (II) to obtain the imatinib (I). The method hasthe advantages that: the process is reasonably designed, expensive reagents are not used, the reaction yield is high, raw materials are low in cost, the operation is simple and convenient, rigorous reaction conditions are absent, and the method is suitable for mass production.
Owner:EAST CHINA UNIV OF SCI & TECH
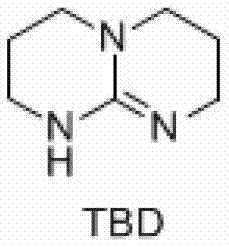
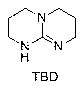
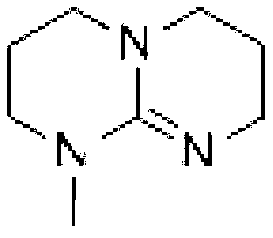
Preparation method of hexahydric dicycloguanidine based on guanidine hydrochloride
ActiveCN103172639AFew synthetic stepsNo harsh reaction conditionsOrganic chemistryDistillationDichlorosilane
The invention discloses a preparation method of hexahydric dicycloguanidine based on guanidine hydrochloride. The preparation method of the hexahydric dicycloguanidine comprises the following steps of: (1), synthesizing TBD (Chloropropyl Methyl Dichlorosilane) by utilizing guanidine hydrochloridel; (2), synthesizing hexahydric dicycloguanidine by utilizing TBD methylation. The preparation method disclosed by the invention is less in synthesis steps, simple and easy to implement, free of severe reaction conditions, capable of adopting a crystallization-distillation purifying method and easy to realize industrial production. The using reagents are low-toxic or non-toxic, and the environment pollution is less. The yields of the two steps are more than 85% and the yields are high. The used materials and reagents are cheap, so that the cost is low.
Owner:RAFFLES PHAMRMATECH CO LTD
Synthetic method for N,N-Ethylene-Bis(bromophthalimide)
InactiveCN102924361AHigh yieldBromination reaction temperature is lowOrganic chemistryEthylenediamineReaction temperature
The invention discloses a synthetic method for N,N-Ethylene-Bis(bromophthalimide). The synthetic method includes the following steps: (1) throwing phthalic anhydride and catalyst into fuming sulphuric acid, dripping bromine in the fuming sulphuric acid at normal temperature to carry out brominating reaction to obtain crude product tetrabromophthalic anhydride, a reaction molar ratio between the phthalic anhydride and the bromine is 1:3.8-4.1; (2) subjecting the crude product tetrabromophthalic anhydride to scouring and complexation, second neutralization and then second water scrubbing to obtain tetrabromophthalic anhydride; (3) Throwing the tetrabromophthalic anhydride into organic solution, dropwise adding ethanediamine diamine on the basis that the molar ratio between the tetrabromophthalic anhydride and the ethidene diamine is 1:1.9-2.1; and obtaining the crude product of N,N-Ethylene-Bis(bromophthalimide) after condensation reaction at the temperature of 50 DEG C to 150 DEGC. (4) Water scrubbing crude product ethylene double bromine phthalates formyl imine to obtain the ethylene double bromine phthalates formyl imine after high-temperature dewatering. The synthetic method is low in temperature of bromination reaction, temperate in reaction condition, easy to control, low in production energy consumption, high in product yield rate, and good in product quality.
Owner:SHANDONG RUNKE CHEM
Thermoplastic high-viscosity high-elasticity asphalt modifier for pavement on steel bridge surface and preparation method thereof
The invention discloses thermoplastic high-viscosity high-elasticity asphalt modifier for pavement on the steel bridge surface and a preparation method thereof. The thermoplastic high-viscosity high-elasticity asphalt modifier comprises, by weight, 40-60% of a thermoplastic elastomer, 2-6% of PE, 15-35% of petroleum resin, 0-25% of a diatomite roasted product, 5-20% of furfural extract oil and 0-6% of an adjuvant. The preparation method comprises adding the thermoplastic elastomer, PE, petroleum resin, diatomite roasted product, furfural extract oil and adjuvant into a spiral extruder and carrying out drawing and granulation under the condition of an extrusion temperature of 120-200 DEG C. The modifier improves asphalt fluidity, high temperature performances and high-viscosity and high-elasticity performances. The preparation method has simple processes, does not need harsh reaction conditions and is convenient for production.
Owner:知行良知实业股份有限公司
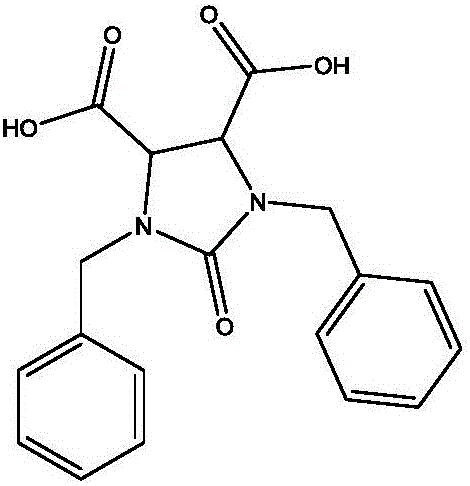
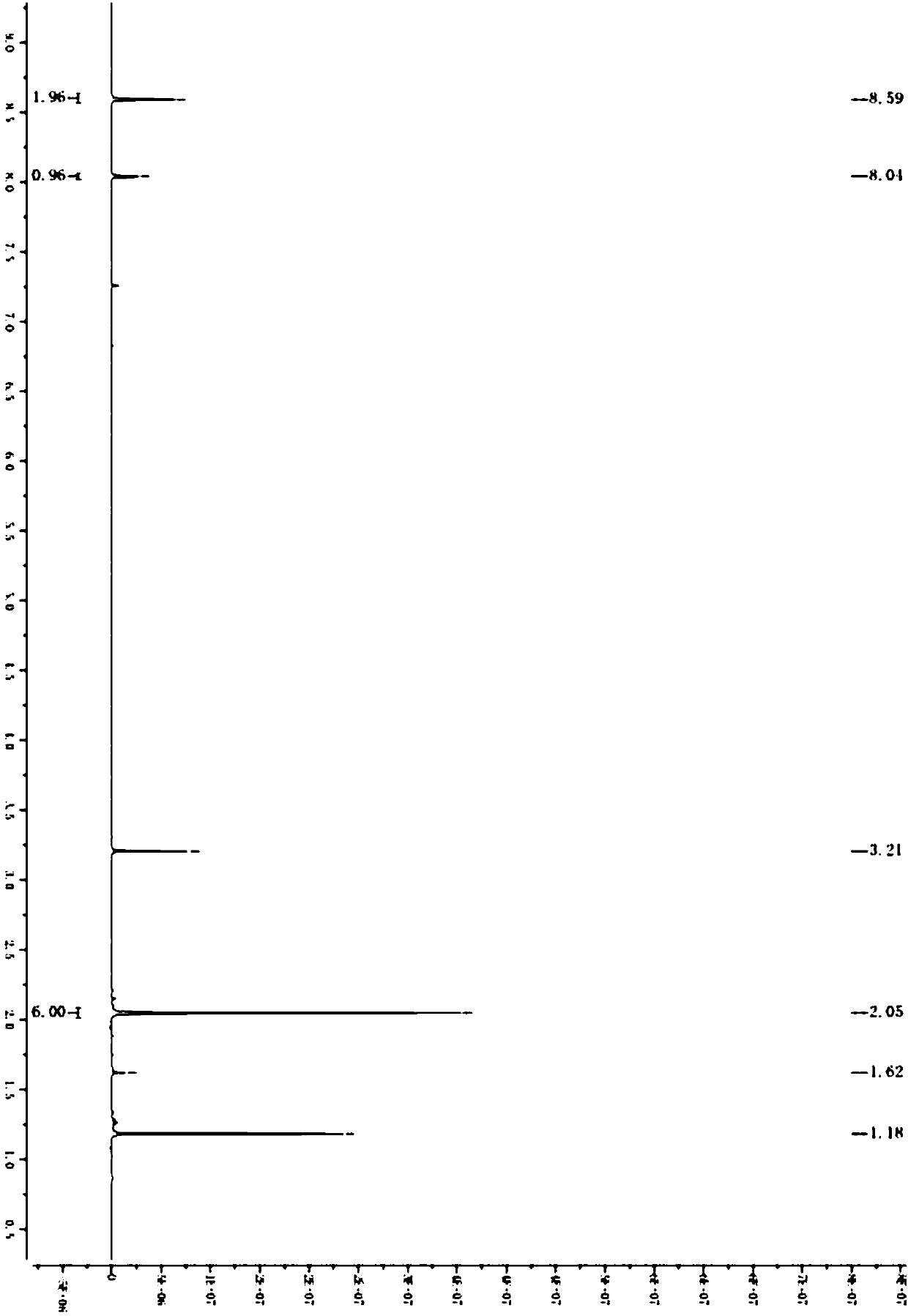
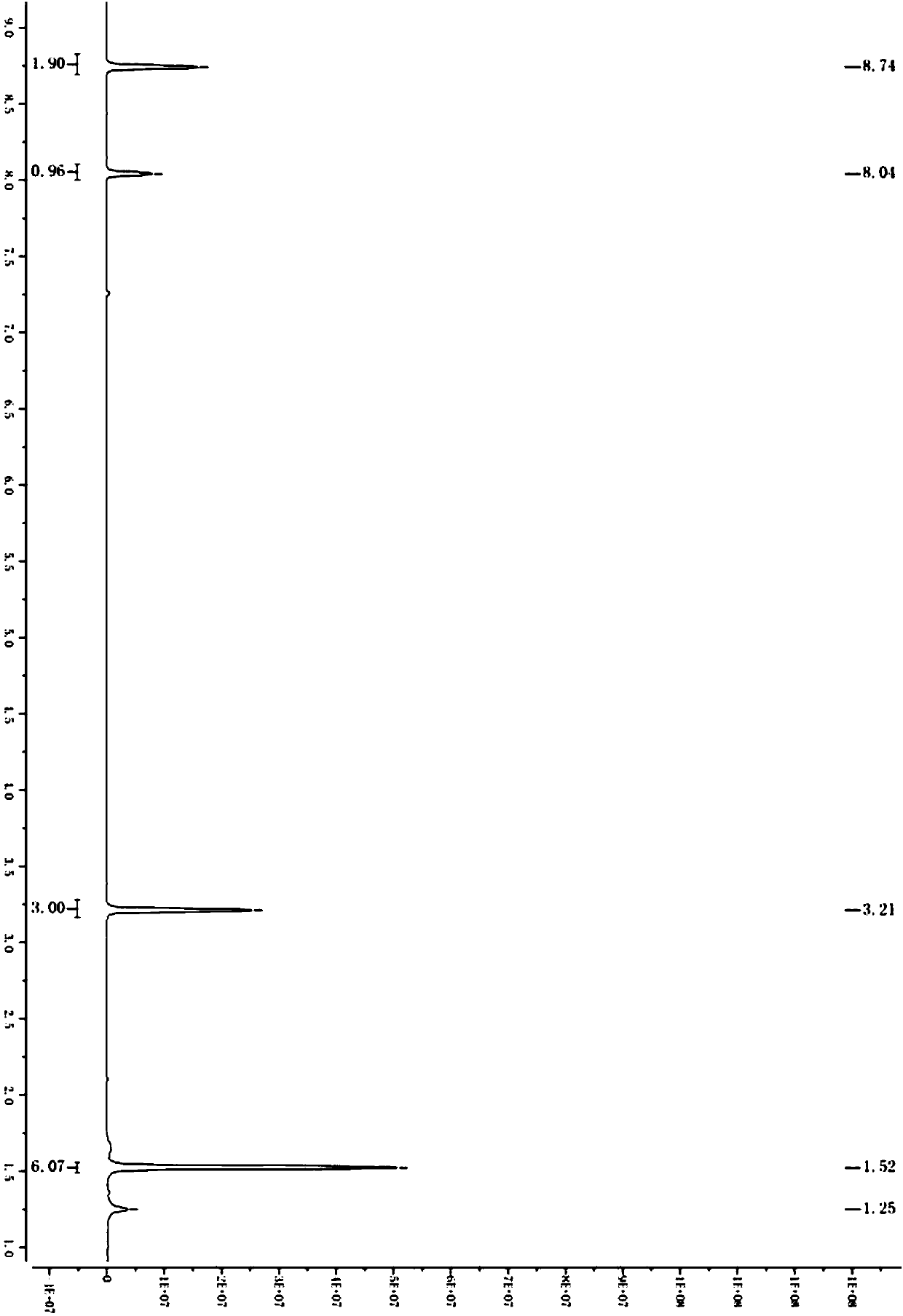
Preparation method of 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid
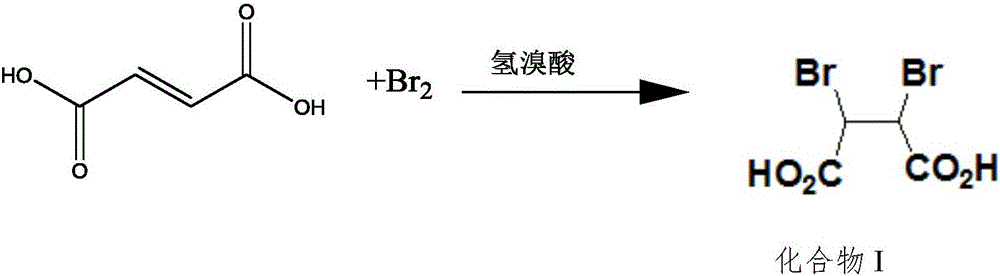
The invention relates to a preparation method of 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid, comprising the steps of subjecting fumaric acid, bromine and hydrobromic acid to additive reaction to generate meso-2,3-dDibromosuccinic acid, subjecting the meso-2,3-dDibromosuccinic acid, benzylamine and a strong base to aminating reaction to generate dibenzylamino salt, subjecting the dibenzylamino salt and triphosgene to cyclization to generate the 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid. The fumaric acid is subjected to addition, and then substitution and cyclization are performed to obtain the 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid, and detection shows that HPLC (high-performance liquid chromatography) content of the 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid may reach 98-99%. The preparation method has the advantages that hydrobromic acid is added for bromination additive reaction, the concentration and reaction temperature of the hydrobromic acid are optimized, and bromine loss is more reduced; amination mother liquid is used in amination, the cost is saved, and product yield is increased; in ring-closure reaction, pH and reaction product concentration are decreased, the yield of the 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid is increased greatly, and the quality of the 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid is improved; experiments verify that through process innovation, production cost is reduced greatly, production efficiency is improved, and the process is energy efficient and environmentally friendly.
Owner:安徽泰格维生素实业有限公司
Method for preparing 2-amino-2-methyl-1-propanol
ActiveCN107129435ARaw materials are cheap and easy to getThe reaction process is simpleOrganic compound preparationPreparation by cyanide reactionPropionitrileSodium cyanide
The invention relates to a method for preparing 2-amino-2-methyl-1-propanol. The method comprises the following steps: 1) enabling 2-chloropropane and sodium cyanide to react to generate 2-methyl propionitrile; 2) performing aldol condensation reaction on the 2-methyl propionitrile and formaldehyde so as to generate 2,2-dimethyl-3-hydroxy propionitrile; 3) performing hydrolysis reaction on 2,2-dimethyl-3-hydroxy propionitrile so as to generate 2,2-dimethyl-3-hydroxy propanamide; and 4) performing Hofmann degradation on the 2,2-dimethyl-3-hydroxy propanamide, thereby obtaining 2-amino-2-methyl-1-propanol. The method is cheap and easy in obtaining of raw materials of different steps, simple in reaction process, free of hash reaction condition, remarkable in cost advantage, high in yield, low in pollution and easy in product purification.
Owner:WANHUA CHEM GRP CO LTD
Preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propioric acid
InactiveCN107721815ALow raw material costShort synthesis processPreparation by organometalhalide reactionOxygen-containing compound preparationBromobenzeneMethyl methacrylate
The invention discloses a preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propioric acid. S1: 3,5-bis(trifluoromethyl)bromobenzene is dissolved in an organic solvent, the solution is added dropwisely into isopropylmagnesium chloride, and a Grignard reagent is obtained; mafosfamide is dissolved in an organic solvent, the solution is added dropwisely into the Grignard reagent, in order to obtain 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propyl-1-ketone; S2: 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propyl-1-ketone obtained in the step S1 is dissolved in an organicsolvent, and 2-(3,5-bis(trifluoromethyl)phenyl)-2-bromo-methyl propyl-1-ketone is obtained; S3: 2-(3,5-bis(trifluoromethyl)phenyl)-2-bromo-2-methyl propyl-1-ketone obtained in the step S2 is dissolved in an organic solvent, zinc bromide is added, in order to obtain 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl methacrylate; S4: 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl methacrylate obtained in the step S3 is dissolved in an organic solvent, the solution is added into an aqueous solution of alkali, and 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propioric acid is obtained.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Synthesizing method of tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether
ActiveCN103193605BSimple methodReduce production energy consumptionOrganic chemistryOrganic compound preparationTetrabromobisphenol AReaction temperature
The invention discloses a synthesizing method of a tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether. The method comprises the following steps: adding tetrabromobisphenol and an alkali into water; stirring until completely dissolving; then carrying out the primary heat preservation; regulating the pH value; adding a dispersing agent; dropwise adding methylallyl chloride and carrying out the secondary heat preservation; after carrying out the primary aftertreatment, obtaining an intermediate product; adding the intermediate product into an organic solvent; stirring until the intermediate product is completely dissolved, and then dropwise adding the bromine; refluxing and carrying out the tertiary heat preservation; and after finishing the tertiary heat preservation, obtaining a product by carrying out the secondary aftertreatment. The method is simple; the adopted solvent can be recycled and reused; the temperature of the bromination reaction is low; the reaction condition is moderate and is easy to control; the energy consumption during the production is low; and the strict reaction conditions are not required by all steps, so that the method is favorable for industrial production. The tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether as the product is high in yield, good in quality, good in heat stability and high in content, thereby lightening the environmental-protection stress. In addition, the tetrabromobisphenol A dual (2,3-dibromo-2-methyl propyl) ether is high in purity, low in impurity content and free of generating 'three wastes' and environmental pollution.
Owner:SHANDONG RUNKE CHEM
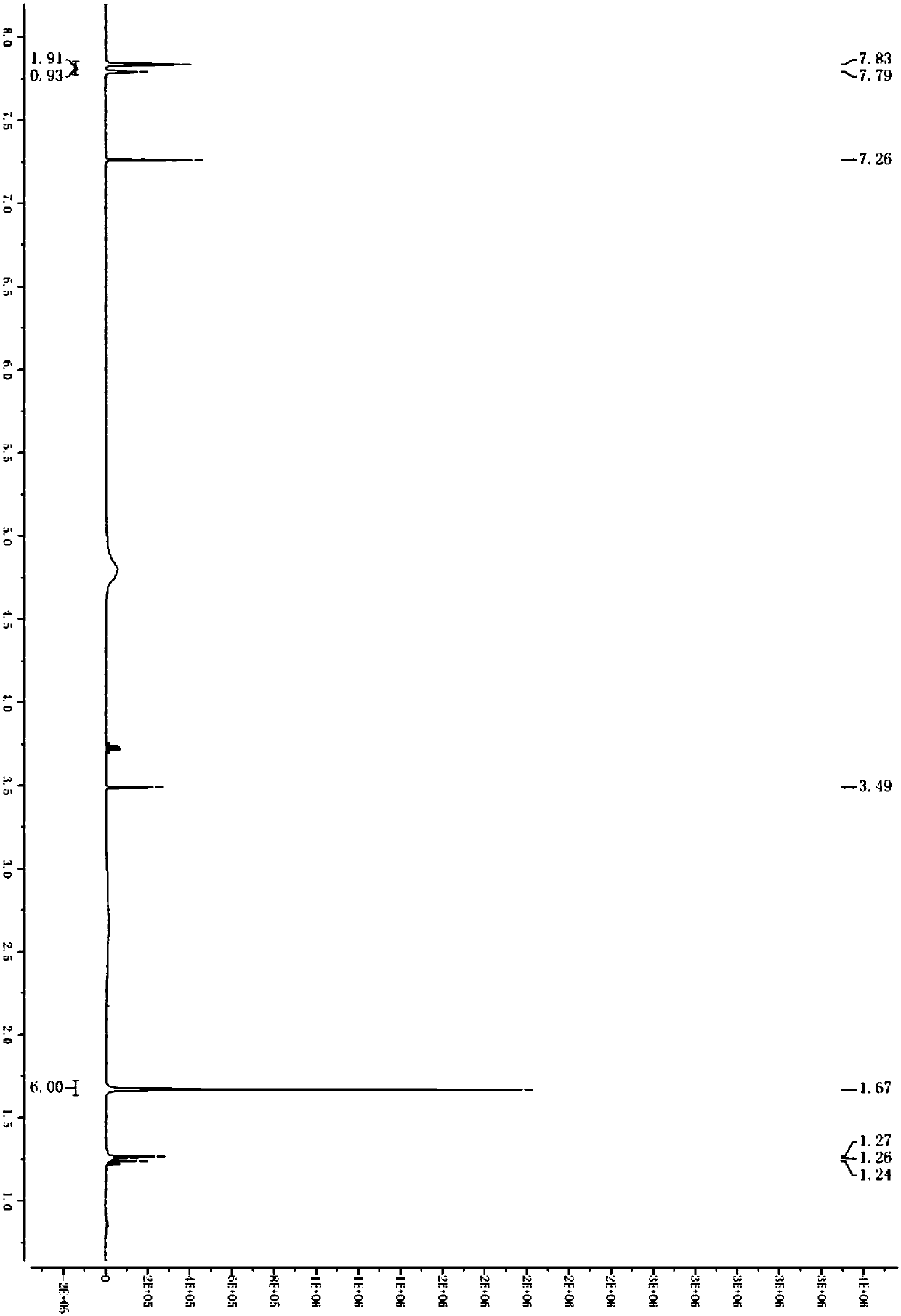
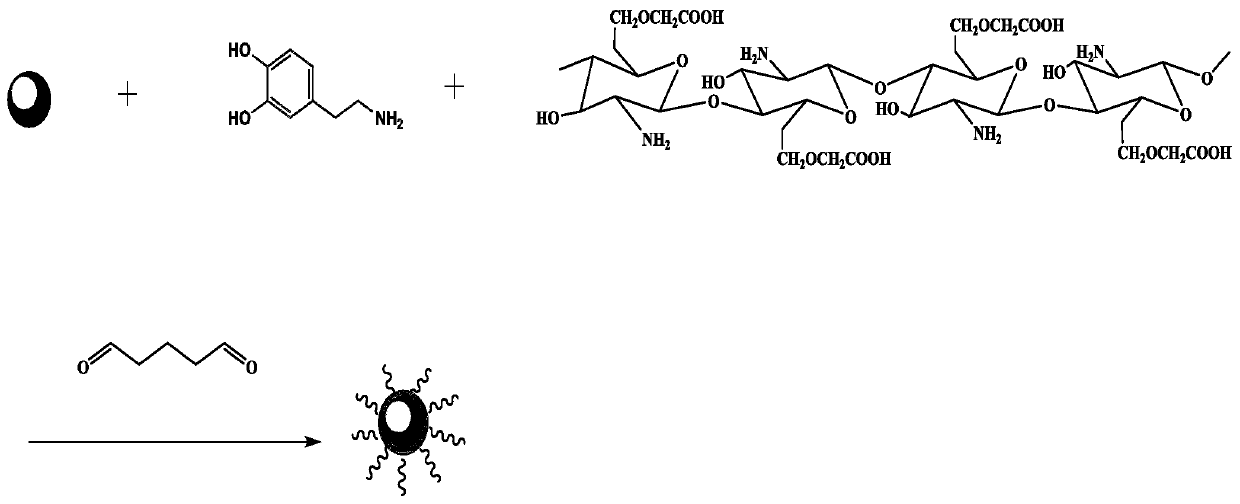
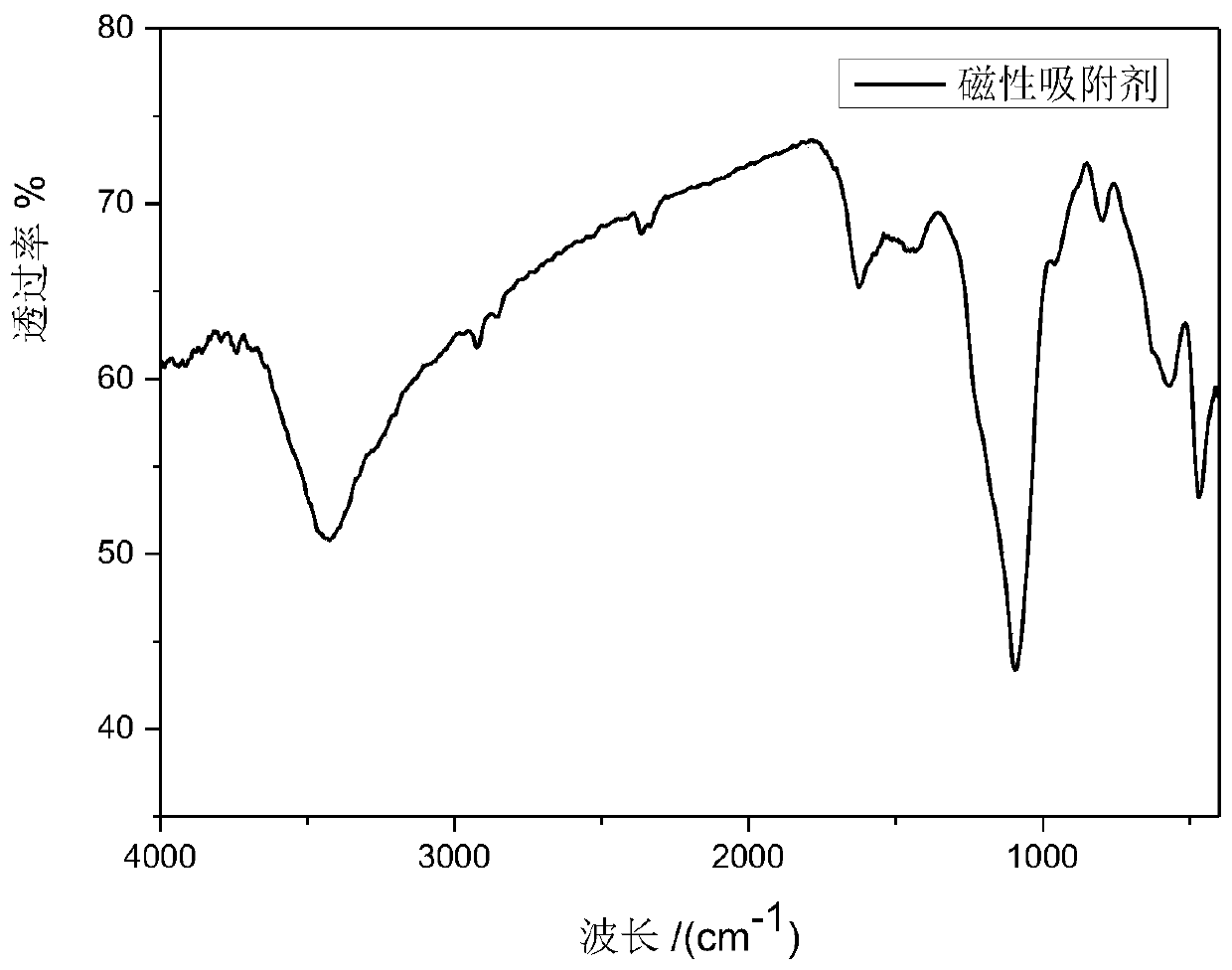
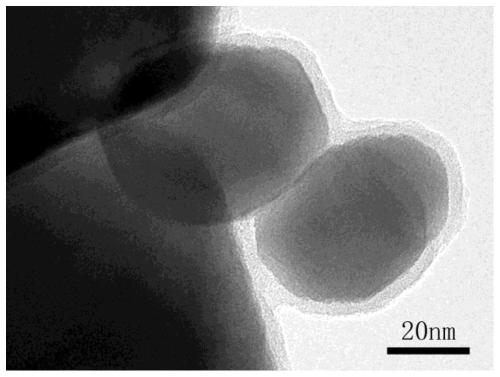
Magnetic polydopamine/carboxymethyl chitosan adsorbent and preparation method and application thereof
InactiveCN110327896ARaw materials are easy to getWide variety of sourcesOther chemical processesWater contaminantsChemistryCoprecipitation
The invention discloses a magnetic polydopamine / carboxymethyl chitosan adsorbent and a preparation method and application thereof. The method comprises the following steps: reacting carboxymethyl chitosan, dopamine and magnetic ferroferric oxide nanoparticles, and then adding a crosslinking agent for reaction; or first reacting the dopamine and the magnetic ferroferric oxide nanoparticles, then adding the carboxymethyl chitosan and the crosslinking agent for reaction; preparing the magnetic polydopamine / carboxymethyl chitosan adsorbent, wherein the magnetic ferroferric oxide nanoparticles areprepared by a coprecipitation method. The magnetic adsorbent prepared by the method has higher adsorption performance on a cationic dye aqueous solution, has large adsorption capacity on a cationic dye aqueous solution, and simultaneously has high-efficiency recycling capability.
Owner:中科广化(重庆)新材料研究院有限公司 +2
Tetrabromo-benzene anhydride diol synthesis method
ActiveCN103232379ASimple processLow reaction temperatureOrganic chemistryDistillationReaction temperature
The invention discloses a tetrabromo-benzene anhydride diol synthesis method comprising the steps that: tetrabromo-benzene anhydride and diethylene glycol are uniformly stirred; a catalyst is added; under the protection of a protective gas, fist heating is carried out, and a reaction is carried out when a reaction temperature is reached; when the reaction is finished, an organic solvent is added for dissolving the reaction material; the temperature is reduced, and epoxypropane is added; secondary heating is carried out, and a maintained-temperature reaction is carried out; after the reaction, hot filtering is carried out; and distillation is carried out for removing the organic solvent, such that the product is obtained. The solvent adopted in the invention can be recovered by distillation and can be recycled, the process is simple, reaction temperature is low, reaction conditions are mild, the reactions are easy to control, production energy consumption is low, no harsh reaction condition is in the steps, industrial production can be easily carried out, product yield is high, product thermal stability is high, product content is high, product quality is good, no three-waste production is caused, and no environment-pollution problem is caused.
Owner:SHANDONG RUNKE CHEM
Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone
InactiveCN108440523ALow costShort synthesis processOrganic chemistryChemical synthesisSynthesis methods
The invention discloses a novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone and relates to the field of chemical synthesis. The method comprises the following steps:by using a four-step synthesis method, performing hydrogen substitution on a benzene ring on 2-amino-3-hydroxymethylpyridine by using bromine so as to generate 2-amino-3-hydroxymethyl-5-bromopyridine;substituting hydroxyl in the 2-amino-3-hydroxymethyl-5-bromopyridine by using chlorine in thionyl chloride so as to generate 2-amino-3-methyl chloride-5-bromopyridine hydrochloride; carrying out an annulation reaction of the 2-amino-3-methyl chloride-5-bromopyridine hydrochloride by using diethyl malonate so as to generate 6-bromine-3-nonanoic acid-ethyl ester-1,2,3,5-tetrahydro-1,8-naphthyridine-2-ketone; finally, under an alkali condition, removing carboxylic acid carbethoxy from the 6-bromine-3-nonanoic acid-ethyl ester-1,2,3,5-tetrahydro-1,8-naphthyridine-2-ketone, thereby obtaining a final product, namely 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone. The method is low in raw material cost, simple in synthesis process, not harsh in reaction condition, safe and convenient to operate, high in final product yield, and applicable to large-scale industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
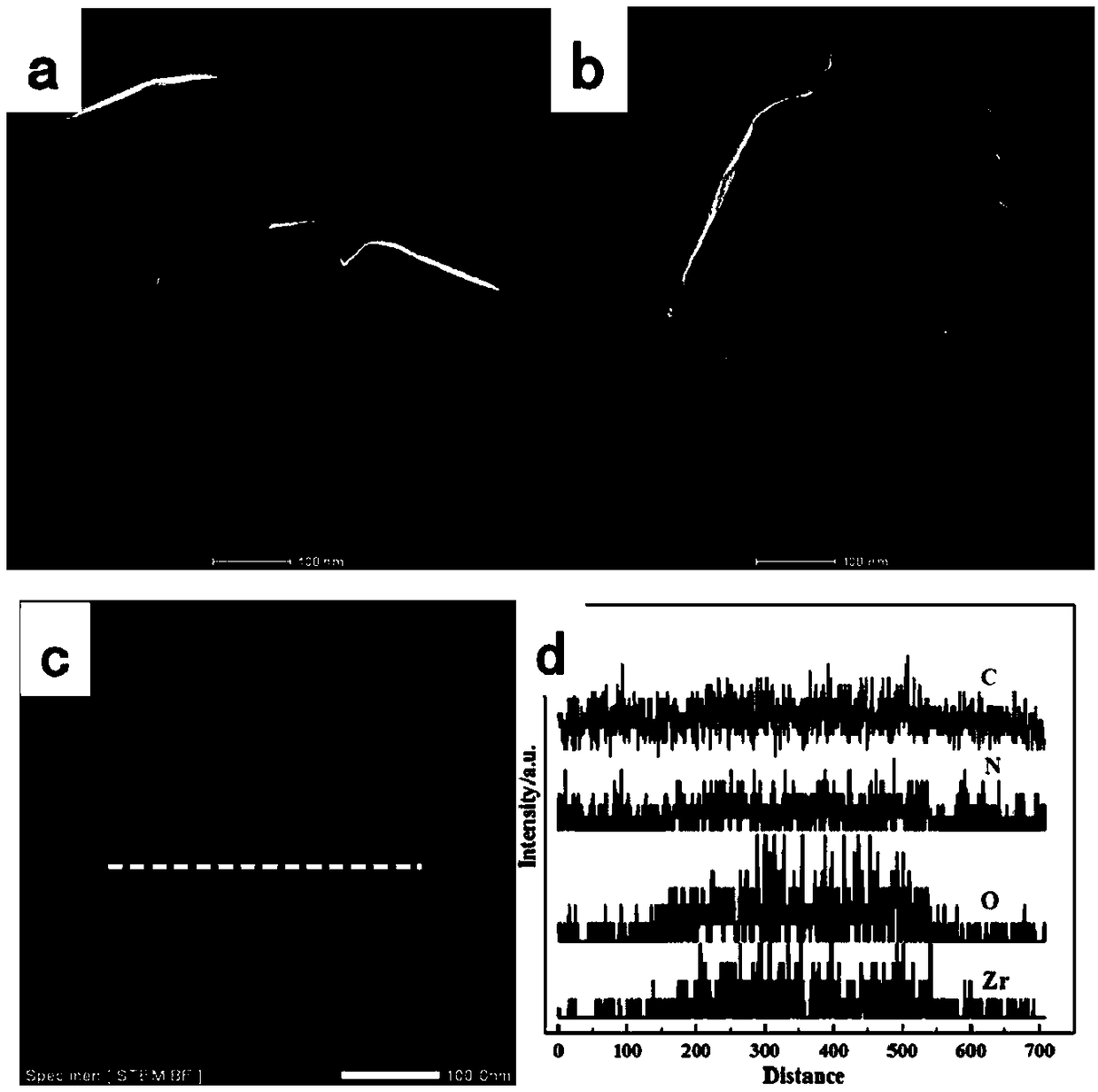
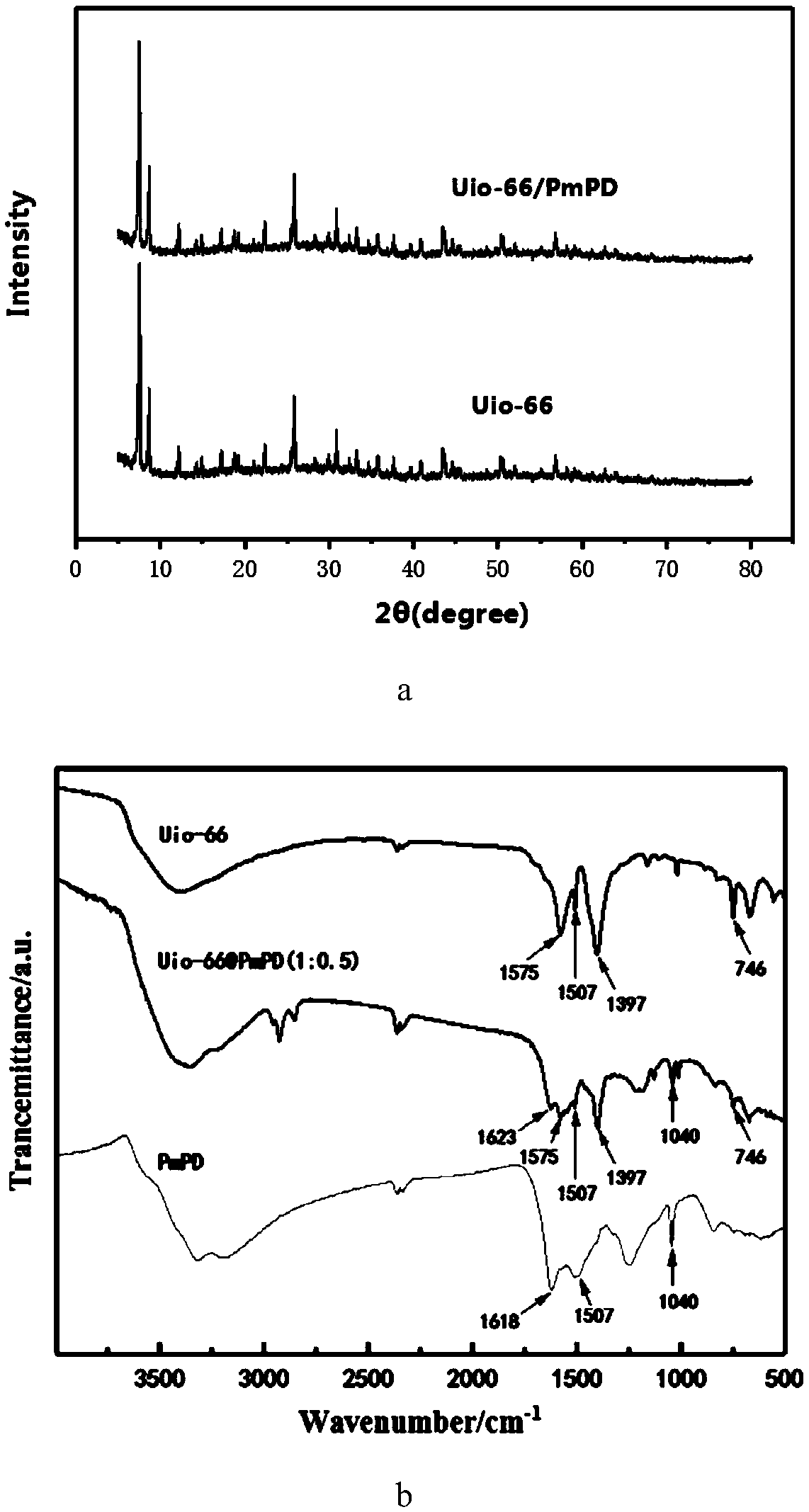
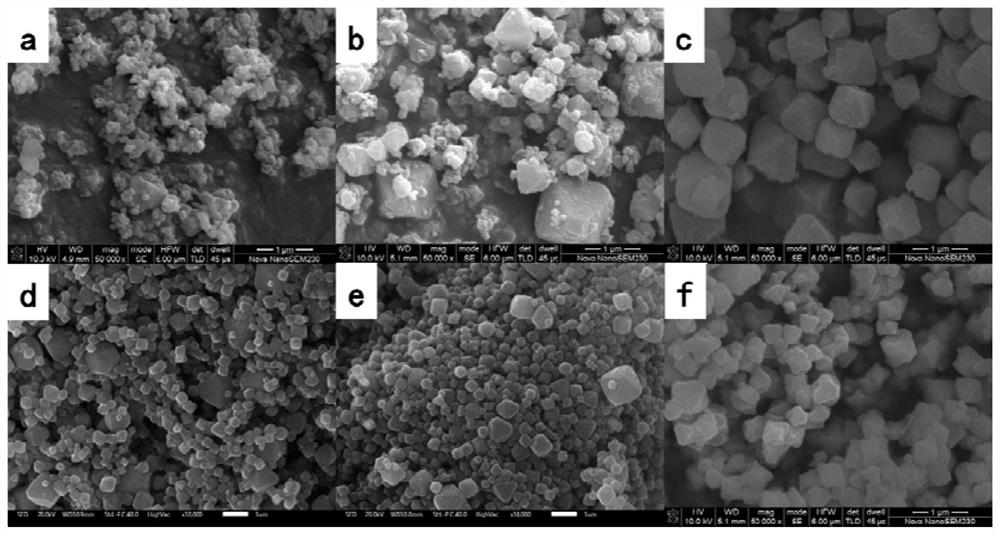
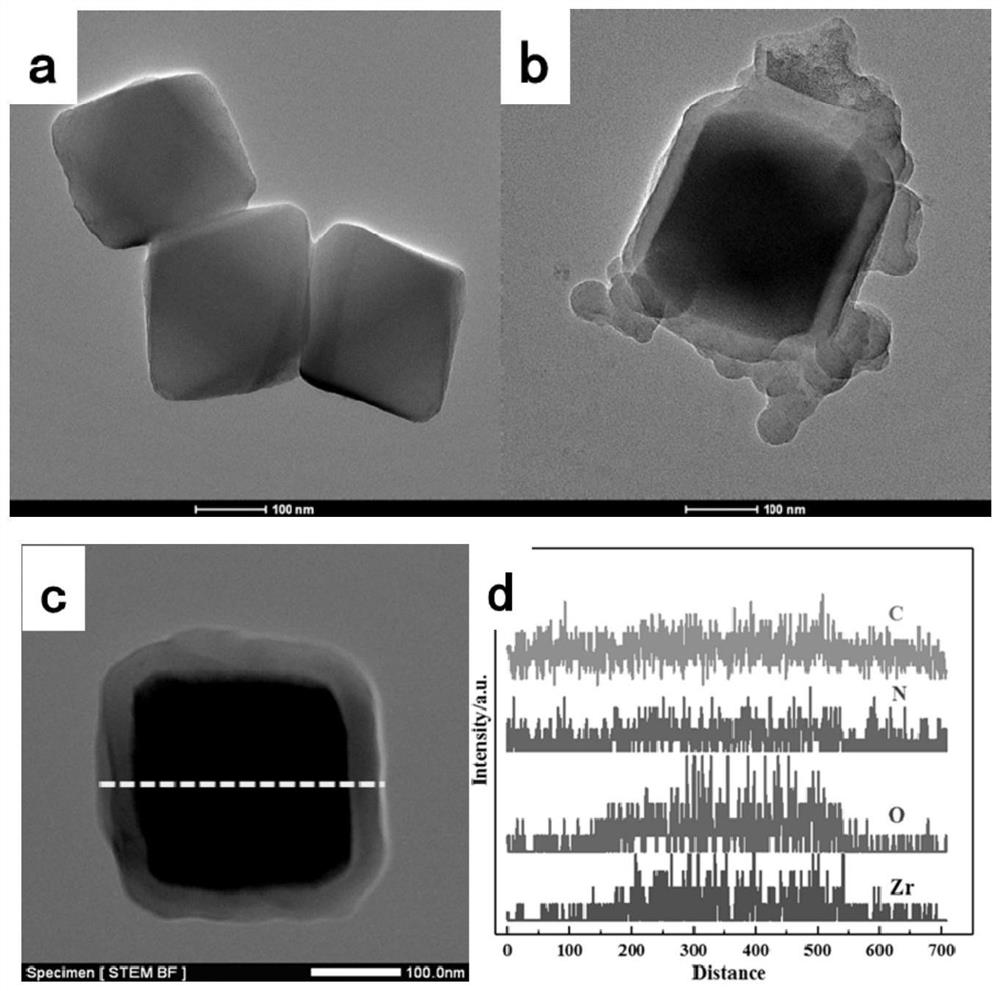
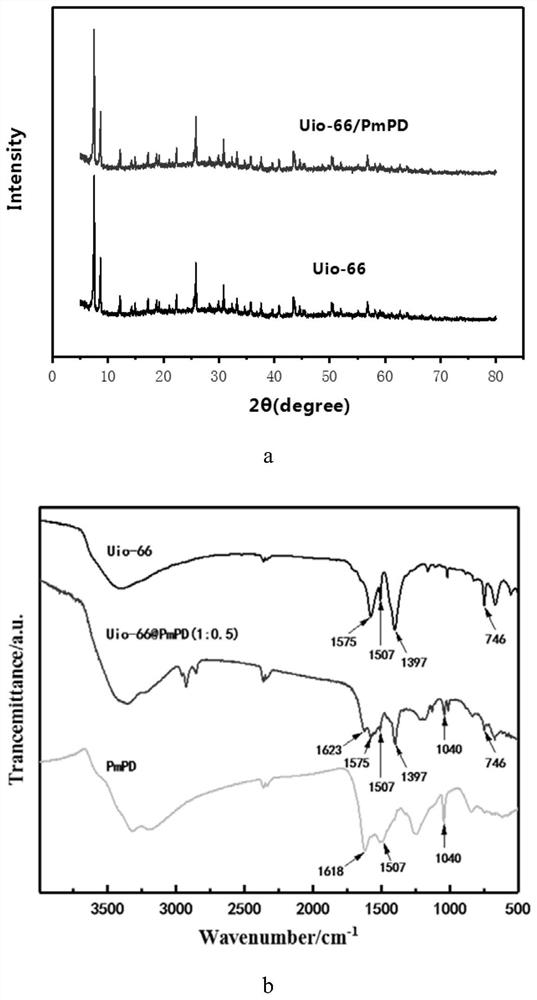
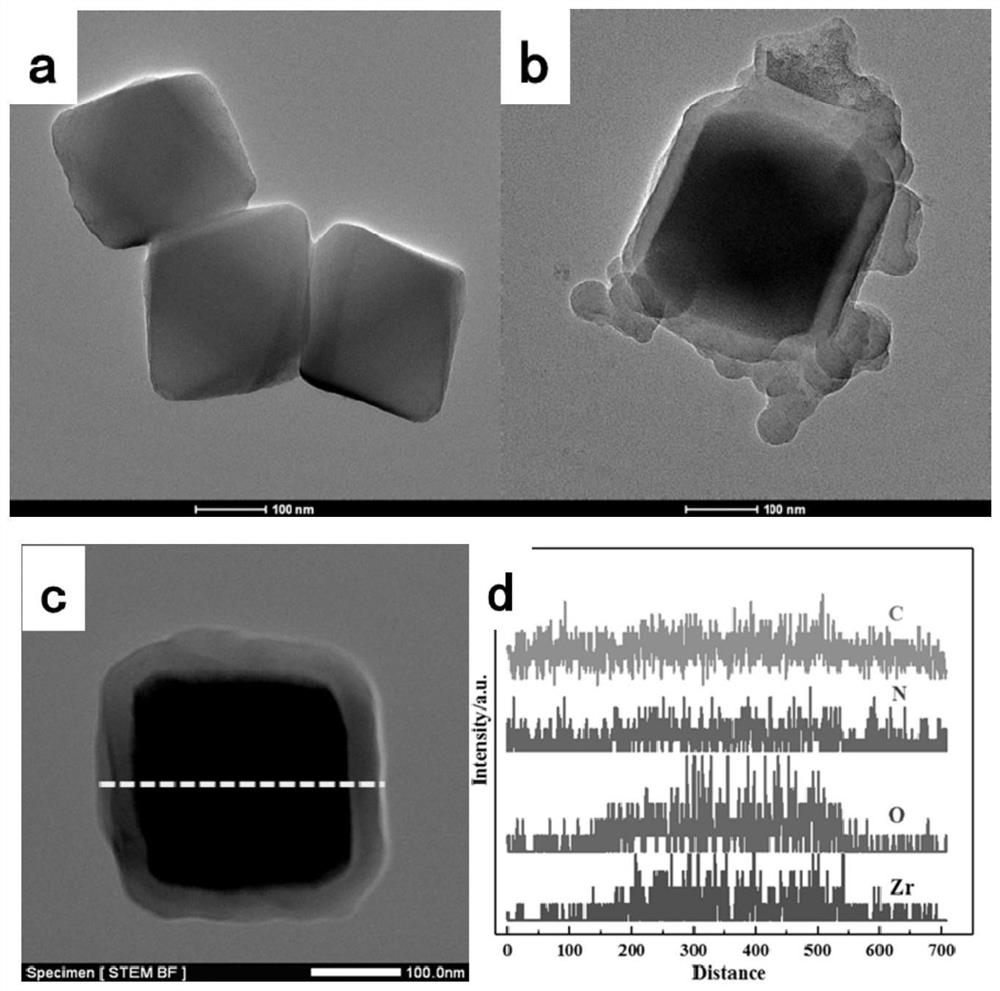
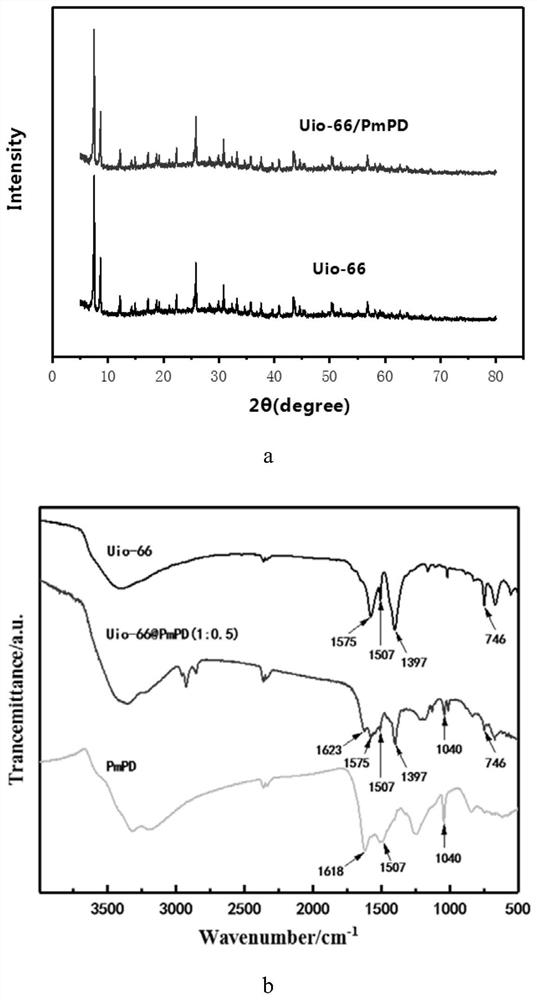
Uio-66/polyaromatic amine composite material with core-shell structure as well as preparation method and application thereof
ActiveCN109054401AHigh specific surface areaImprove Oxidative Polymerization EfficiencyOther chemical processesWater contaminantsSulfateAqueous solution
The invention discloses a Uio-66 / polyaromatic amine composite material with a core-shell structure as well as a preparation method and application thereof. The preparation method comprises the following steps: dispersing Uio-66 in an aqueous solution; adding an aromatic amine monomer and a surfactant, and stirring for 0.5-24 h for sufficient contact; dropwise adding a sulfate aqueous solution to initiate a polymerization reaction, and continuously stirring for 3-24 h; performing centrifugal separation, and washing to obtain a Uio-66 / polyaromatic amine composite material. The specific surface area of the composite material reaches 319.77m<2> / g, thereby effectively increasing the specific surface area of polyaromatic amine; moreover, the surface of Uio-66 is subjected to amination modification successfully. The method disclosed by the invention can be used for efficiently preparing the Uio-66 / polyaromatic amine composite material, and is economical and effective and easy to operate, andthus has a broad application prospect in the technical field of water treatment.
Owner:CENT SOUTH UNIV
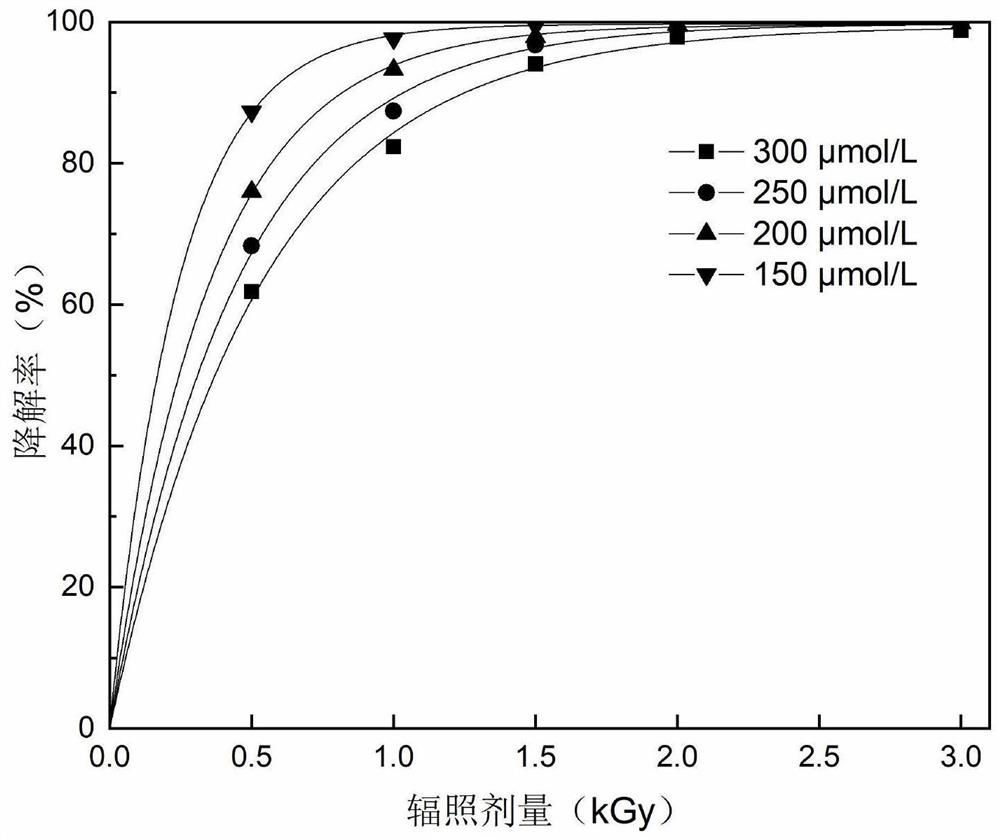
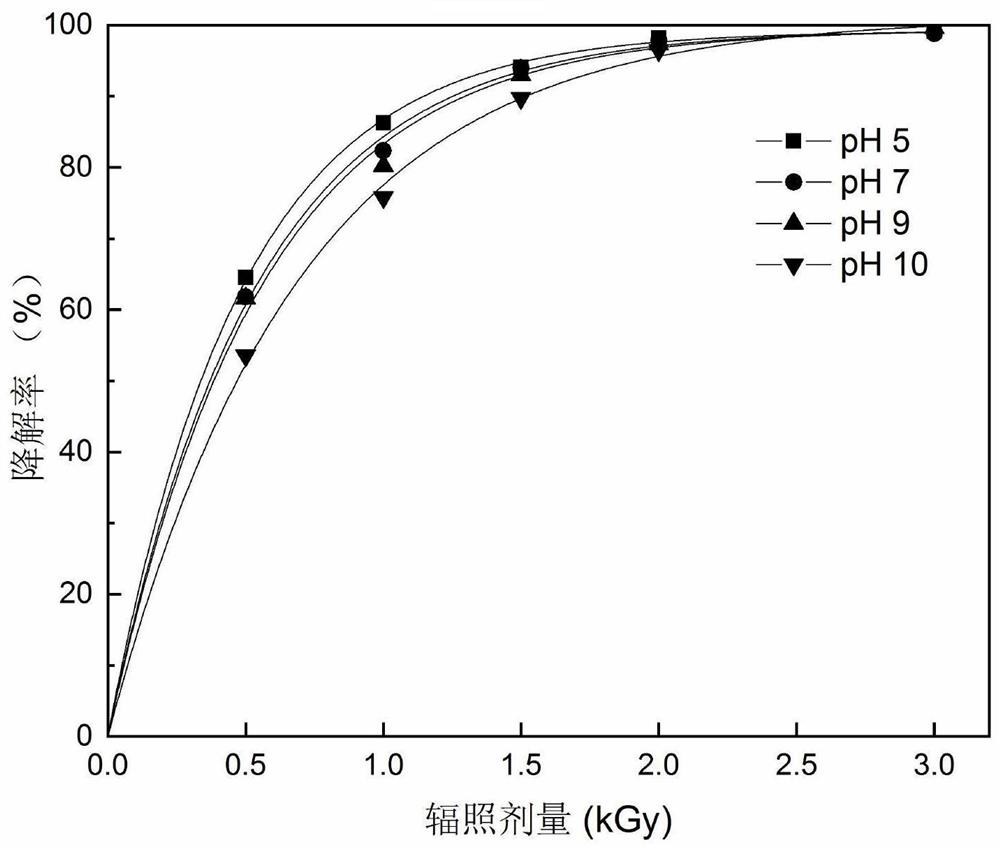
Method for degrading anti-inflammatory drug indometacin in water body by utilizing electron beam irradiation
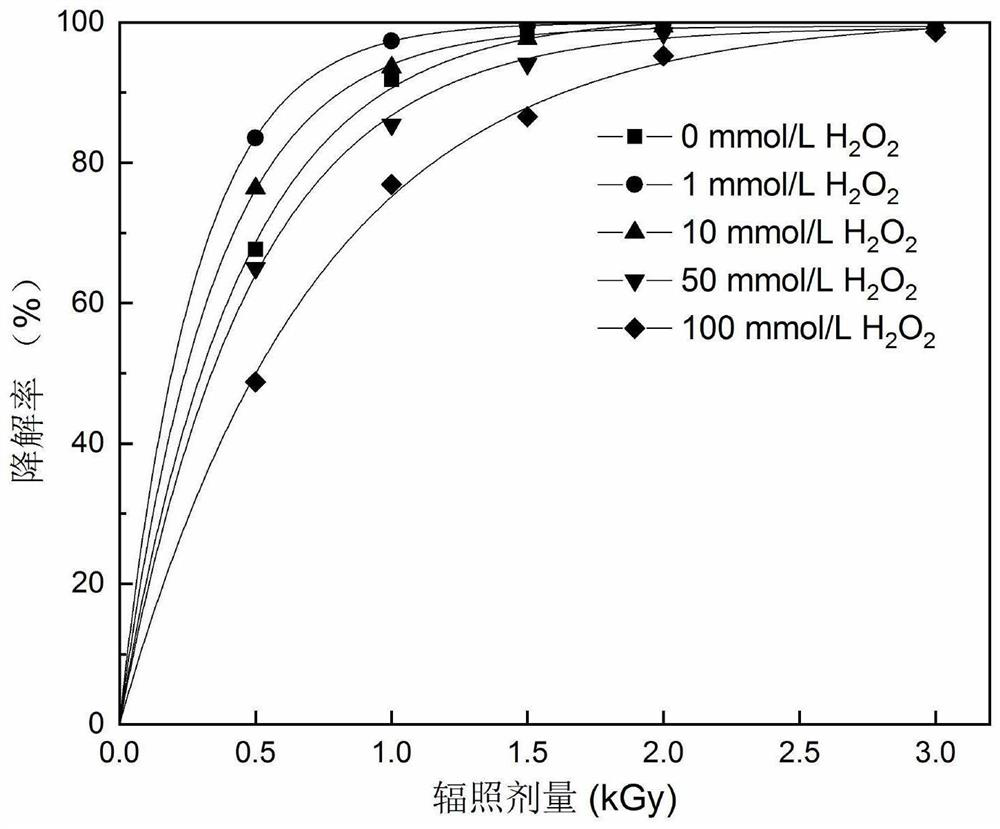
PendingCN111718041AEasy to operatePromotes free radical productionWater/sewage treatment by irradiationWater treatment compoundsAntiinflammatory drugPollutant
The invention discloses a method for degrading an anti-inflammatory drug indometacin in a water body by utilizing electron beam irradiation, and belongs to the field of water treatment and environmental protection. The water decomposition is excited by electron beam irradiation to generate .OH and H. with high reactivity, and indometacin is degraded under the oxidation and reduction actions, so that the method is a simple and efficient pollutant removal method. An indometacin solution with a certain concentration is subjected to irradiation treatment under an electron beam, the irradiation dose is 0.5-3 kGy, the removal effect is improved along with the increase of the dose, the method conforms to quasi-primary dynamics, and indometacin can be completely removed in a short time. 1-100 mmol / L of hydrogen peroxide is added into the solution, so that irradiation degradation of indometacin can be promoted, and the economy of irradiation treatment of pollutants is improved. The method is high in removal efficiency, high in reaction rate, simple and convenient to operate, free of secondary pollution and wide in applicability.
Owner:SHANGHAI UNIV
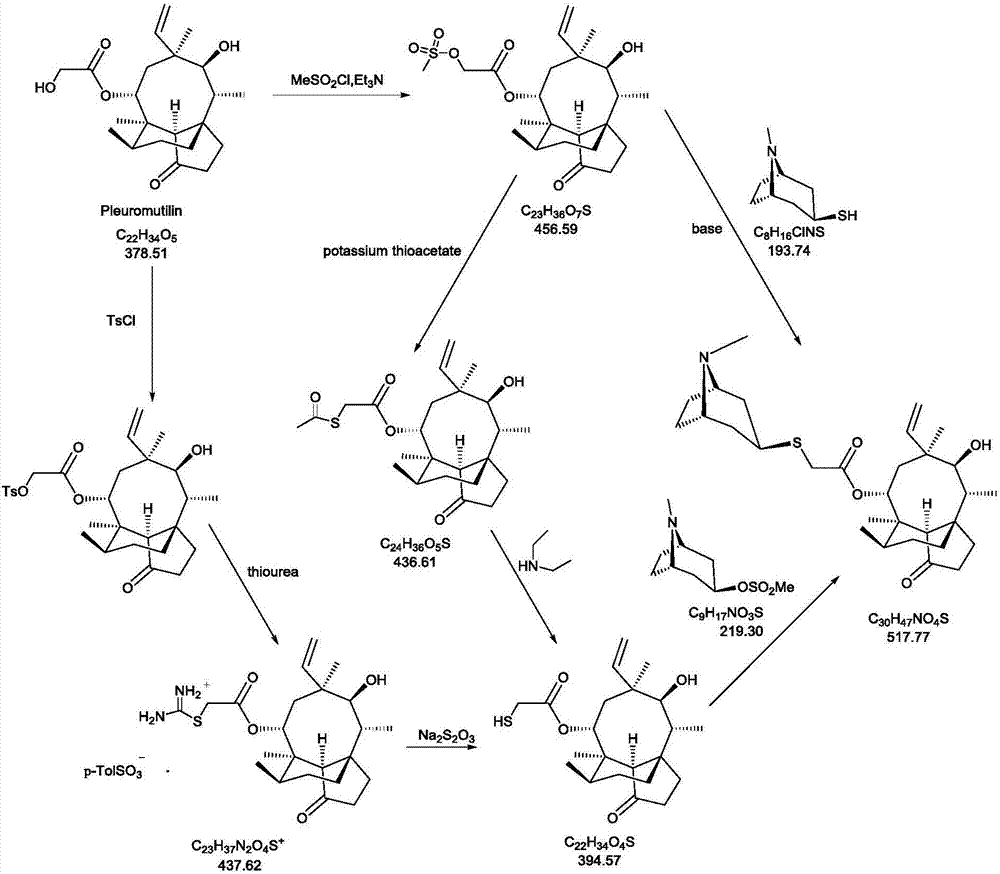
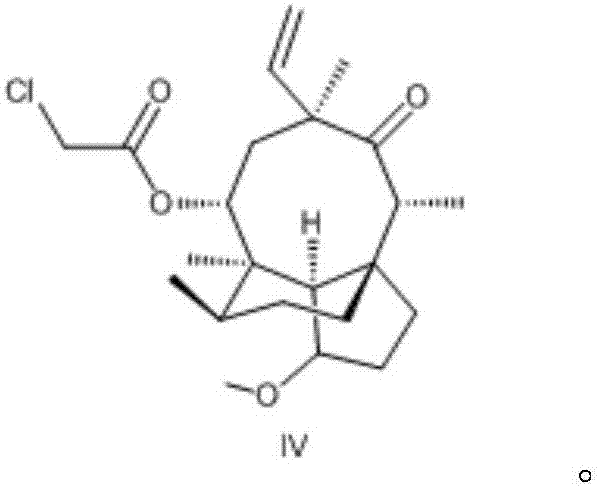
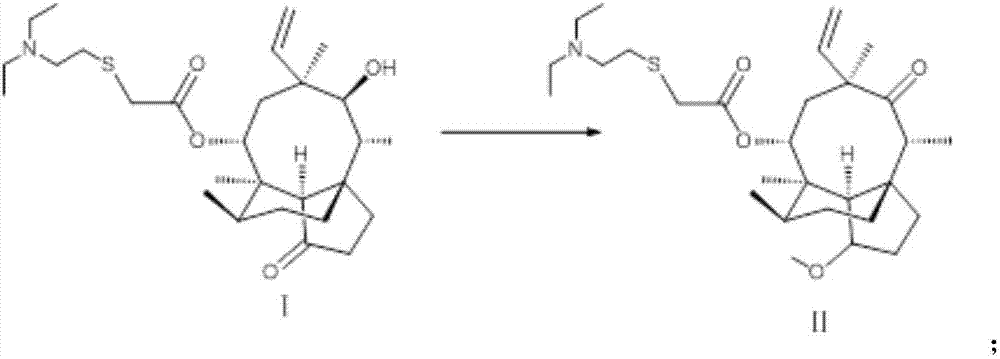
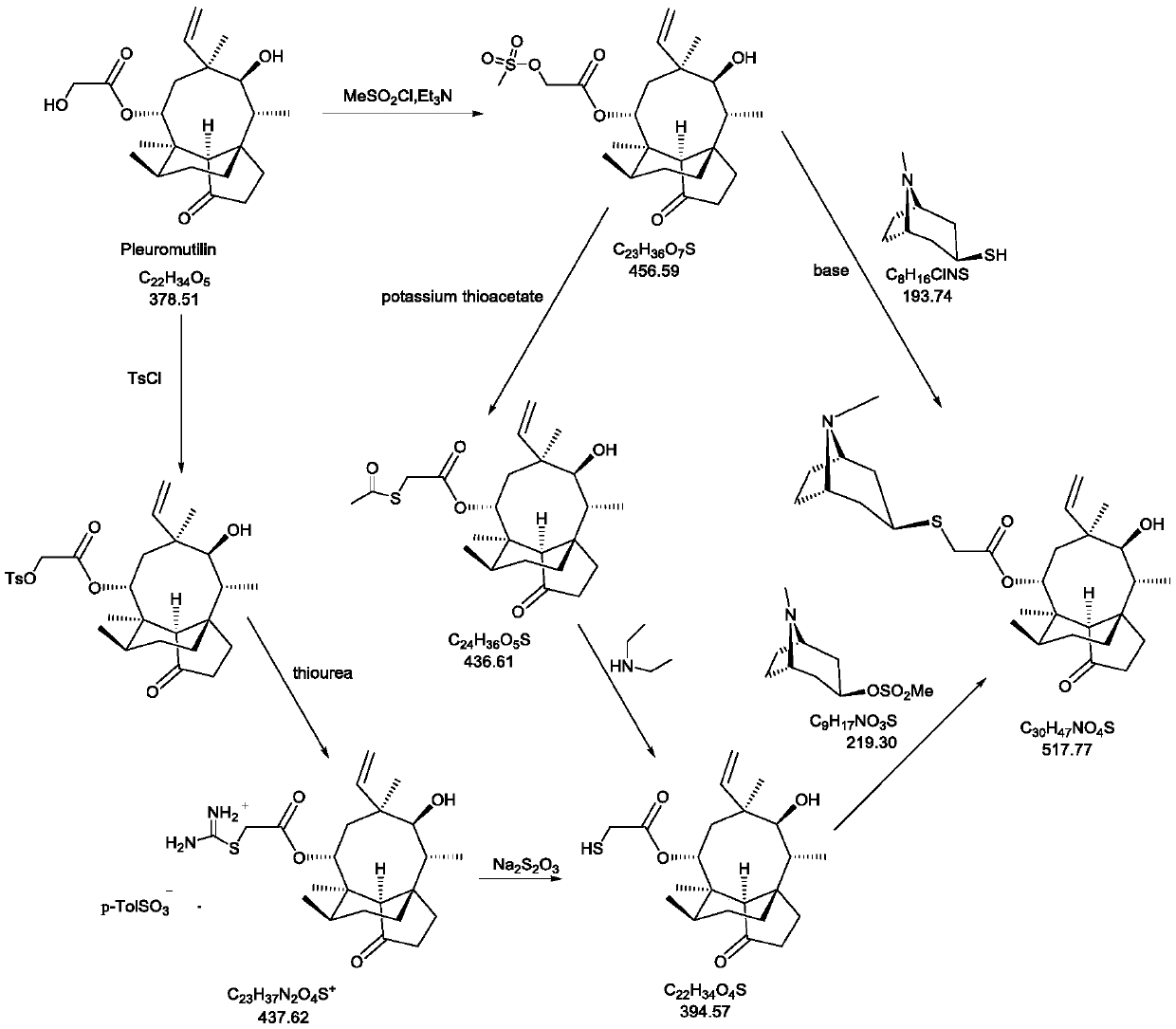
Novel method for preparing external-use antibiotic drug retapamulin
ActiveCN107324998AImpurity spectrum is clearSimple process operationOrganic compound preparationCarboxylic acid esters preparationAntibiotic DrugsRetapamulin
The invention provides a novel method for preparing an external-use antibiotic drug retapamulin. The novel method comprises the following steps: taking tiamulin as a starting raw material, and preparing retapamulin through five-step solid chemical reaction and a refining process. According to the novel method provided by the invention, quality of the starting raw material is controllable, so that the novel method is suitable for novel drug development and declaration. The novel method is simple to operate, is controllable in condition, is low in preparation cost, is convenient for industrial production, and is significant innovation for a retapamulin synthesis method.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Water-based adhesive and preparation method thereof, as well as battery
InactiveCN106398597AEfficient manufacturingEasy to operateNon-macromolecular adhesive additivesCell electrodesWater basedOrganic solvent
The invention provides a water-based adhesive and a preparation method thereof, as well as a battery. The water-based adhesive is prepared from polypropylene emulsion with carboxyl, a non-ionic thickening agent, a conductive agent, a resistance reducing agent and water. The adhesive does not contain toxic volatile organic solvents; the water is used as a medium, so that the physical environment is friendly, and the adhesive is safe to a worker; glue formed by the non-ionic thickening agent is high in stability; furthermore, by adoption of the polypropylene emulsion with carboxyl, the adhesive has reaction activity; after the adhesive is cured, an electrode is high in size stability.
Owner:BAIC MOTOR CORP LTD
Application of a uio-66/polyaromatic composite material
ActiveCN108855010BLarge specific surface areaEasy to manufactureOther chemical processesWater contaminantsEnvironmental engineeringAqueous solution
The invention discloses application of a Uio-66 / polyaromatic composite material in treating wastewater containing hexavalent Cr. The Uio-66 / polyaromatic composite material is characterized in that anadsorbent is dissolved into an aqueous solution and an aromatic amine monomer is formed on the surface of Uio-66 through an in situ chemical oxidation polymerization method. The prepared Uio-66 / polyaromatic composite material has the advantages of simple synthesis process, low cost, high yield, good removal effect on Cr(VI) in water and no secondary pollution. At room temperature, the maximum adsorption capacity of the adsorbent can reach 263.69mg / g, and the removal rate can reach 99 percent or above which is higher than that of non-composite Uio-66 and majority reported chromium adsorption materials. Acidic wastewater containing Cr(VI) is treated by adopting the method disclosed by the invention; the method has the characteristics of simple process, convenience in operation, good economicbenefits and the like.
Owner:CENT SOUTH UNIV
A kind of method for preparing topical antibiotic drug retapamulin
ActiveCN107324998BQuality is easy to controlFacilitate quality researchOrganic compound preparationCarboxylic acid esters preparationChemical reactionBiochemical engineering
The invention provides a novel method for preparing an external-use antibiotic drug retapamulin. The novel method comprises the following steps: taking tiamulin as a starting raw material, and preparing retapamulin through five-step solid chemical reaction and a refining process. According to the novel method provided by the invention, quality of the starting raw material is controllable, so that the novel method is suitable for novel drug development and declaration. The novel method is simple to operate, is controllable in condition, is low in preparation cost, is convenient for industrial production, and is significant innovation for a retapamulin synthesis method.
Owner:CHONGQING HUABANGSHENGKAI PHARM
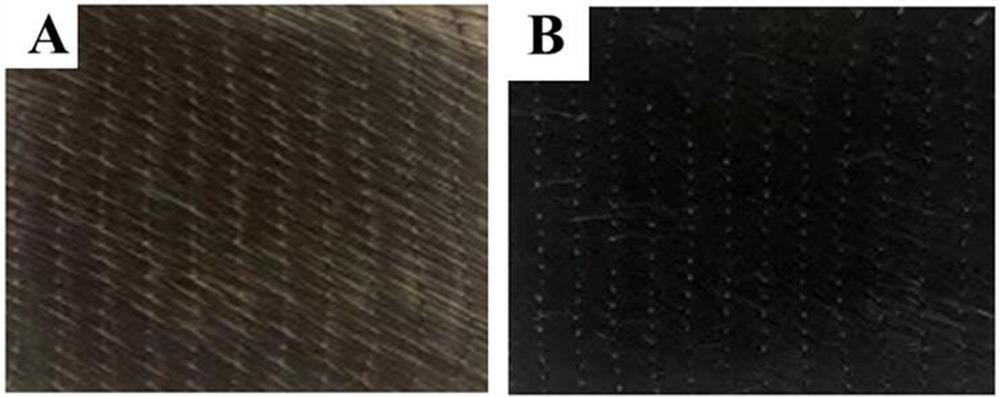
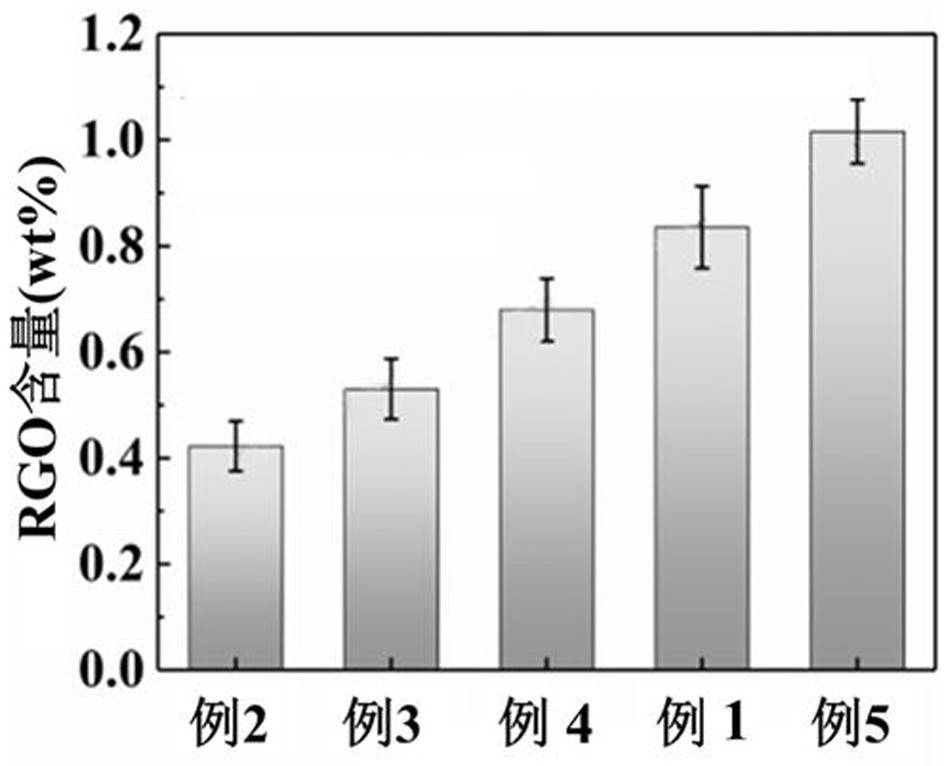
Simplified preparation method of graphene conductive fiber cloth and broadband electromagnetic wave absorption composite material thereof
ActiveCN114575148AFacilitate the transfer of chargeImprove the ability of interface polarization loss electromagnetic waveGeneral water supply conservationMagnetic/electric field screeningBroadbandGraphite oxide
The invention relates to a simplified preparation method of graphene conductive fiber cloth and a broadband electromagnetic wave absorption composite material thereof. Graphene nanosheets are uniformly loaded on the surface of glass fiber cloth by adopting a layer-by-layer self-assembly matched chemical reduction process and are mutually lapped to form a conductive layer. All the graphene oxide physically adsorbed on the surface of the glass fiber cloth is removed by controlling ultrasonic power and time, so that the loaded graphene oxide uniformly wraps the surface of the glass fiber cloth through hydrogen bond interaction between the loaded graphene oxide and the glass fiber cloth and pi-pi action between sheet layers. The method is simple and easy to implement, green and environmentally friendly, and large-scale production is easy to achieve; in addition, under the condition that the graphene content is extremely low, good conductivity and very excellent wave absorbing performance are achieved. The graphene conductive fiber cloth is used as a reinforcing body to be compounded with a polymer matrix, and the structural wave-absorbing composite material which is thin in thickness, high in absorption strength and wide in absorption band can be obtained.
Owner:ZHONGBEI UNIV +1
A kind of method for preparing 2-amino-2-methyl-1-propanol
ActiveCN107129435BRaw materials are cheap and easy to getThe reaction process is simpleOrganic compound preparationPreparation by cyanide reactionPropionitrile1-Propanol
The present invention relates to a kind of method for preparing 2-amino-2-methyl-1-propanol, comprising the following steps: 1) 2-chloropropane and sodium cyanide react to generate 2-methylpropionitrile; 2) 2-methylpropionitrile 2,2-dimethyl-3-hydroxypropionitrile undergoes an aldol condensation reaction with formaldehyde; 3) 2,2-dimethyl-3-hydroxypropionitrile undergoes a hydrolysis reaction to generate 2,2-dimethyl 4) 2,2-dimethyl-3-hydroxypropionamide undergoes Hofmann degradation reaction to generate 2-amino-2-methyl-1-propanol. The raw materials in each step of the method are cheap and easy to obtain, the reaction process is simple, there are no harsh reaction conditions, the cost advantage is obvious, the yield is high, the pollution is low, and the product is easy to purify.
Owner:WANHUA CHEM GRP CO LTD
Preparation method of sitagliptin and intermediate of sitagliptin
ActiveCN103319487BLow priceStrong chiral induction abilityOrganic chemistrySitagliptinMedicinal chemistry
The invention belongs to the field of pharmaceutical chemistry, and more specifically relates to sitagliptin which is used for treatment of type 2 diabete and an intermediate of sitagliptin. The invention provides a compound represented by formula II. The invention also discloses applications of the compound which is represented by formula II in preparation of sitagliptin. Sitagliptin is obtained by catalytic hydrogenation of the compound. The method of the invention can be used to produce sitagliptin simply and conveniently, and is low in cost, and is easy to industrialize.
Owner:2Y CHEM
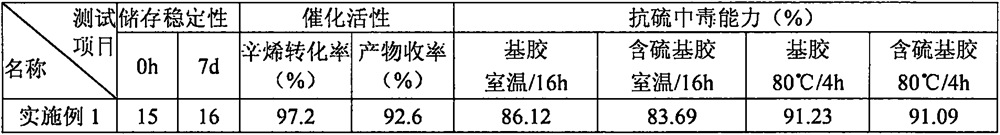
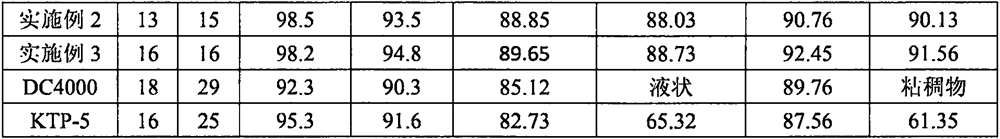
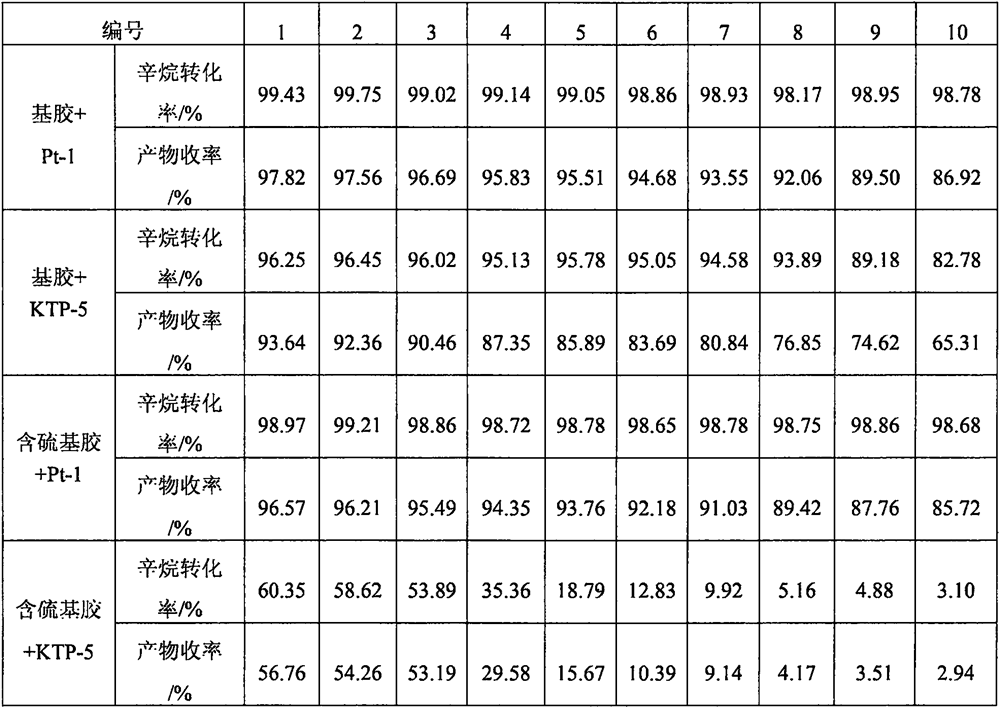
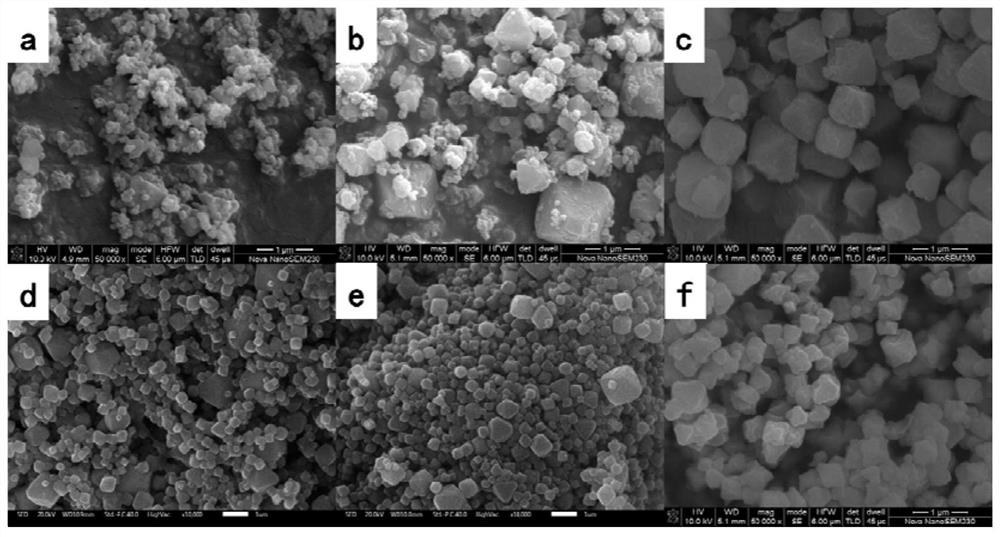
Anti-sulfur poisoning platinum catalyst and preparation method thereof
The invention discloses an anti-sulfur-poisoning platinum catalyst and a preparation method thereof, the preparation method comprises the following steps: A, a carrier material is dispersed into a 0#solvent, amino alkoxyl silane is added for mixing, an acid catalyst and water are added, and the mixture is in reaction to obtain a modified carrier material; B, the solvent, epithio-heterocyclic monomers and modified platinum dichloride (II) are mixed and stirred, an acid stabilizer and an acid catalyst are added, and water is added dropwise for reaction to obtain a modified platinum catalyst; C,under the condition that the inert gas is fed, the solvent, the modified carrier material and a modified platinum catalyst solution are mixed, warming reaction is conducted, reaction is performed under the vacuumizing condition, then the temperature is reduced to 0 DEG C sharply and standing is performed, and the anti-sulfur-poisoning platinum catalyst is obtained after separation. According to the platinum catalyst, the catalytic activity and the stability are improved remarkably, the poisoning effect is reduced greatly when the platinum catalyst is used in synthesis of sulfur-containing silicone oil and silicon resin, and the anti-poisoning effect is remarkable.
Owner:SHENZHEN ANPIN SILICONE MATERIAL
A kind of uio-66/polyarylamine composite material with core-shell structure and its preparation method and application
ActiveCN109054401BLarge specific surface areaImprove Oxidative Polymerization EfficiencyOther chemical processesWater contaminantsPolymer scienceActive agent
Owner:CENT SOUTH UNIV
A kind of preparation method of six-membered bicyclic guanidine based on guanidine hydrochloride
ActiveCN103172639BFew synthetic stepsNo harsh reaction conditionsOrganic chemistryDistillationDichlorosilane
The invention discloses a preparation method of hexahydric dicycloguanidine based on guanidine hydrochloride. The preparation method of the hexahydric dicycloguanidine comprises the following steps of: (1), synthesizing TBD (Chloropropyl Methyl Dichlorosilane) by utilizing guanidine hydrochloridel; (2), synthesizing hexahydric dicycloguanidine by utilizing TBD methylation. The preparation method disclosed by the invention is less in synthesis steps, simple and easy to implement, free of severe reaction conditions, capable of adopting a crystallization-distillation purifying method and easy to realize industrial production. The using reagents are low-toxic or non-toxic, and the environment pollution is less. The yields of the two steps are more than 85% and the yields are high. The used materials and reagents are cheap, so that the cost is low.
Owner:RAFFLES PHAMRMATECH CO LTD
A kind of synthetic method of tetrabromophthalic anhydride diol
ActiveCN103232379BSimple processLow reaction temperatureOrganic chemistryDistillationSynthesis methods
The invention discloses a method for synthesizing tetrabromophthalic anhydride diol, comprising the following steps: stirring tetrabromophthalic anhydride and diethylene glycol evenly, adding a catalyst, and under the protection of protective gas, heating up to the reaction temperature for the first time After the reaction is completed, an organic solvent is added to dissolve the above-mentioned reaction materials, propylene oxide is added after the temperature is lowered, the temperature is raised for the second time to carry out a heat preservation reaction, and after the reaction is completed, hot filtration is performed, and then the organic solvent is distilled off to obtain the product. The solvent used in the reaction of the method of the invention can be re-evaporated and applied mechanically, the process is simple, the reaction temperature is low, the reaction conditions are mild, easy to control, and the production energy consumption is low, no harsh reaction conditions are required in each step, and industrial production is easy, and the product yield is high. , good thermal stability, high content, good product quality, and there is no generation of three wastes, there is no problem of environmental pollution.
Owner:SHANDONG RUNKE CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone](https://images-eureka.patsnap.com/patent_img/d30798b0-033b-4288-af64-a0d75926f928/RE-FDA0001656230280000011.png)
![Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone](https://images-eureka.patsnap.com/patent_img/d30798b0-033b-4288-af64-a0d75926f928/RE-FDA0001656230280000012.png)
![Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone Novel method for synthesizing 6-bromine-3,4-dihydro-1H-[1,8] naphthyridine-2-ketone](https://images-eureka.patsnap.com/patent_img/d30798b0-033b-4288-af64-a0d75926f928/RE-FDA0001656230280000013.png)