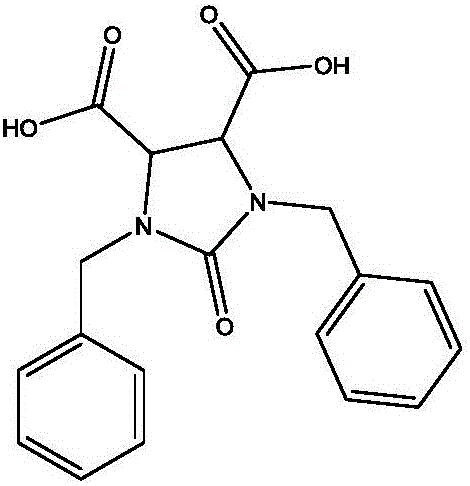
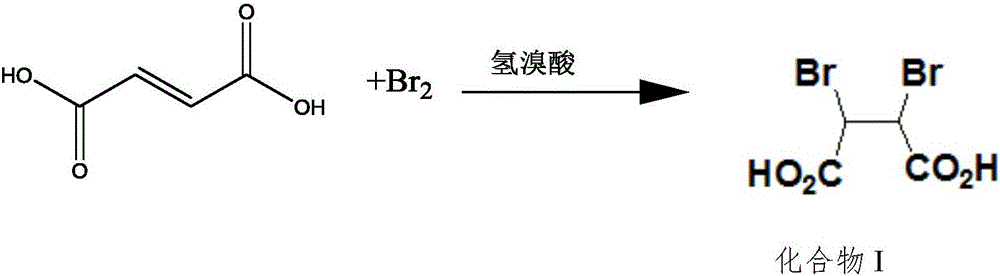
Preparation method of 1,3-dibenzyl-2-oxoimidazolidine-4,5-dicarboxylic acid
A cyclic acid and fumaric acid technology, applied in the direction of organic chemistry, can solve problems such as control difficulties, and achieve the effects of optimizing reaction temperature, short synthesis route and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of cyclic acid, comprising the following steps:
[0046] 1) The addition reaction of bromination generates dibromosuccinic acid adduct:
[0047] Add 60g of fumaric acid, 70g of water, and 12g of concentrated hydrobromic acid (5.5% by mass) into the reaction flask, raise the temperature to 60-70°C, add 80g of bromine dropwise, keep a certain pressure in the reaction flask, and raise the temperature to 70 ~90°C, keep warm for 5-10 hours, cool down, crystallize, and filter to obtain compound I (ie, dibromosuccinic acid adduct).
[0048] 2) Amination substitution reaction to generate dibenzylamide potassium salt
[0049]Put 60g of compound I (dibromosuccinic acid adduct) and 250ml of amination mother liquor into the reaction flask, control the temperature at 20-50°C, add 129g of benzylamine dropwise, and control the temperature at 20-50°C during the dropping process, Raise the temperature to 70-90°C, keep it warm for 5-10 hours, cool down, filter, add...
Embodiment 2
[0053] A preparation method of cyclic acid, the only difference from Example 1 is: step 2) the amination substitution reaction uses about 250ml of the amination mother liquor produced in the previous batch; the obtained dibenzylamide potassium salt HPLC content is 99.5%, and the yield The rate is 85%.
Embodiment 3
[0055] A preparation method of cyclic acid, the difference from Example 1 is only: step 1) adopts mass fraction 5.5% hydrobromic acid to stabilize the reaction concentration in the bromination addition reaction; the prepared dibromosuccinic acid HPLC content is 99% %, the yield is 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com