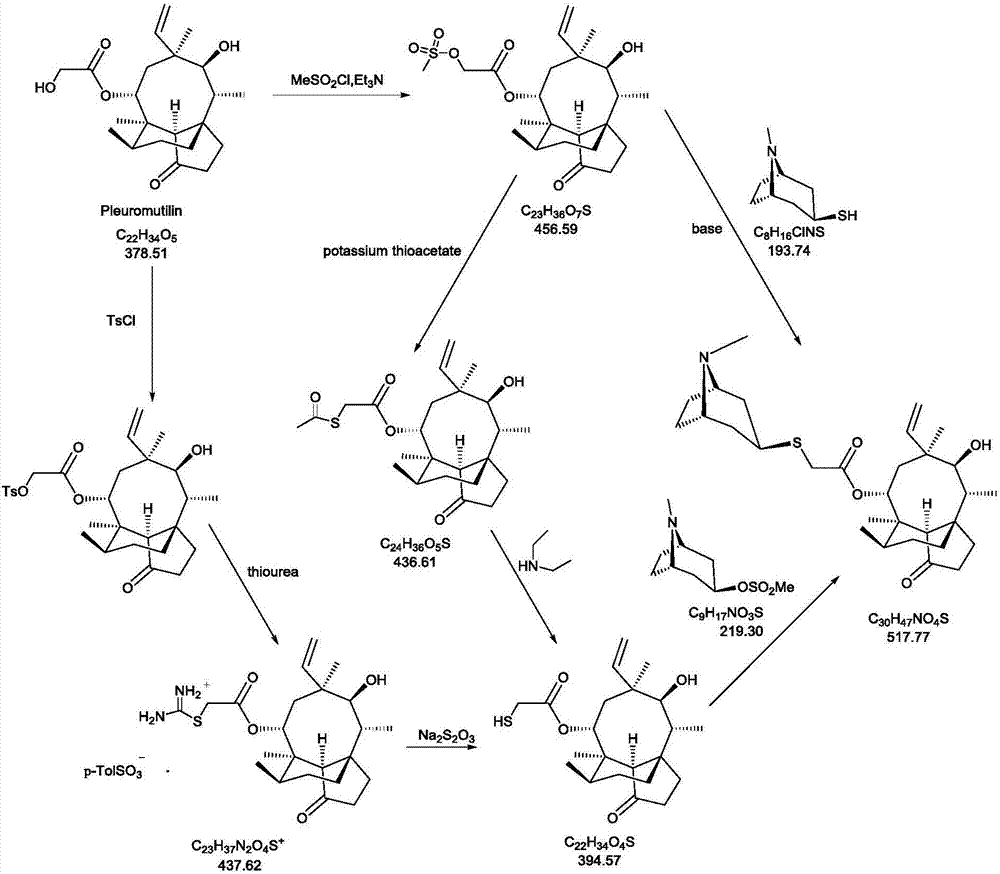
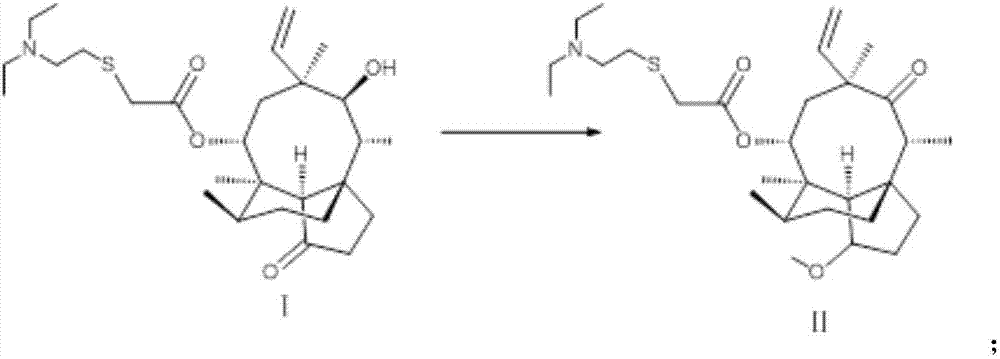
Novel method for preparing external-use antibiotic drug retapamulin
A retamoline and system technology, which is applied in the new field of preparation of topical antibiotic drug retamoline, can solve the problems of increasing impurity research by drug manufacturers, affecting the quality of retamoline products, and complex impurity spectrum of fermentation products. , to achieve the effect of convenient industrial production, clear impurity spectrum and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of the compound shown in embodiment 1 formula III
[0064] The chemical name of the compound shown in formula III is (3aS, 4R, 6S, 8R, 9R, 9aR, 10R)-4,6,9,10-tetramethyloctahydro-8-hydroxyl-1-methoxy-6 - Vinyl-3a,9-propane-3aH-cyclopentacyclooctan-5-one.
[0065] Add 1.22kg of the compound of formula I and 7.5L of anhydrous methanol into a 50L double-layer glass reactor, under nitrogen protection, stir to dissolve, then add 1.18kg of trimethyl orthoformate, cool down; start adding concentrated sulfuric acid dropwise at 0-10°C, Keep warm at <15°C, drop it for about 1.0-1.5 hours, a total of 602.9g of concentrated sulfuric acid; heat up to an internal temperature of 45-50°C to react, and the reaction is completed in about 6.0-7.0 hours, stop heating, lower the temperature to 10-20°C, and start dripping Add 50% aqueous sodium hydroxide solution, control temperature <30°C, drop in about 1.0 hour, add 2.17kg of 50% aqueous sodium hydroxide solution dropwise;...
Embodiment 2
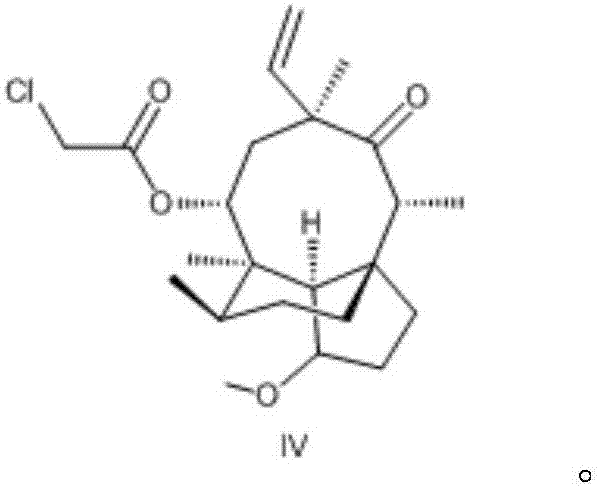
[0066] The preparation of embodiment 2 formula IV compound
[0067] The chemical name of the compound of formula IV is (3aS, 4R, 6S, 8R, 9R, 9aR, 10R)-4,6,9,10-tetramethyloctahydro-8-hydroxy-1-methoxy-6-ethylene Base-3a,9-propane-3aH-cyclopentacyclooctane-5-one-8-chloroacetate.
[0068] Add 2.4L of methyl tert-butyl ether and 210.0g of the compound of formula III into a 10L double-layer glass reactor, stir to dissolve, and the stirring speed is 200-300 rpm; the high and low temperature cycle machine cools down, and the temperature is controlled at -10 to - Pyridine (223.0g in total) was added dropwise at 15°C, and the dropwise addition was completed in about 15-20 minutes; the temperature was lowered, and the temperature was controlled at -20~-25°C, and a mixture of chloroacetyl chloride (146.0g) and 750ml methyl tert-butyl ether was added dropwise Liquid, a large amount of yellow-white solids are formed in the reaction kettle, and the dropwise addition is completed in about ...
Embodiment 3
[0069] The preparation of embodiment 3 formula V compound
[0070] The chemical name of the compound of formula V is (3aS, 4R, 5S, 6S, 8R, 9R, 9aR, 10R)-4,6,9,10-tetramethyloctahydro-5,8-hydroxyl-6-vinyl- 3a,9-Propane-3aH-cyclopentacyclooctan-1-one-8-chloroacetate.
[0071] Add 315.0g of the compound of formula IV and 1.58L of acetonitrile into a 10.0L reaction kettle, slowly drop into concentrated hydrochloric acid solution of zinc chloride (312.7g of zinc chloride dissolved in 945mL of concentrated hydrochloric acid) under temperature control at 5-15°C with stirring, about After 1.5-2.0 hours, the dropwise addition was completed, and then the temperature was raised to 40.0-50.0°C to react for 1.0-1.5 hours. After the reaction was completed, 2.0L of drinking water was added to quench the reaction, extracted with dichloromethane (2.5L*2), and the organic phases were combined and used for drinking Wash with water (3.0L*2); dry the organic phase with 1.0kg of anhydrous sodium s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com