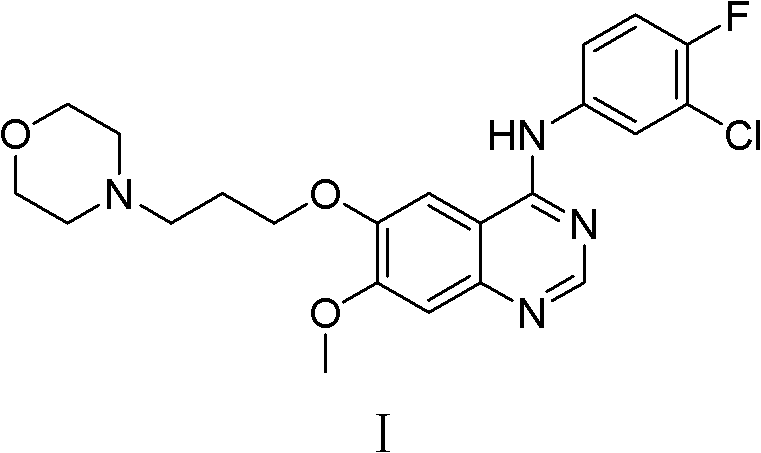
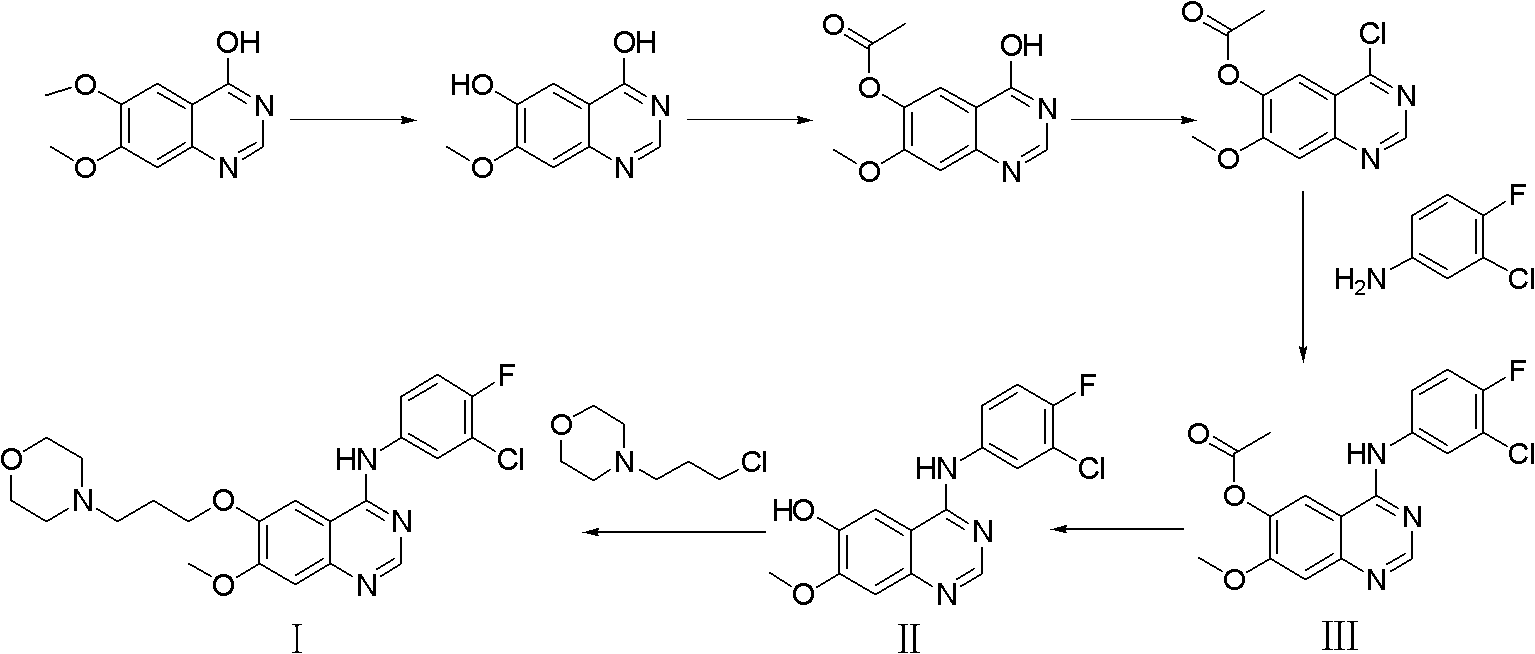
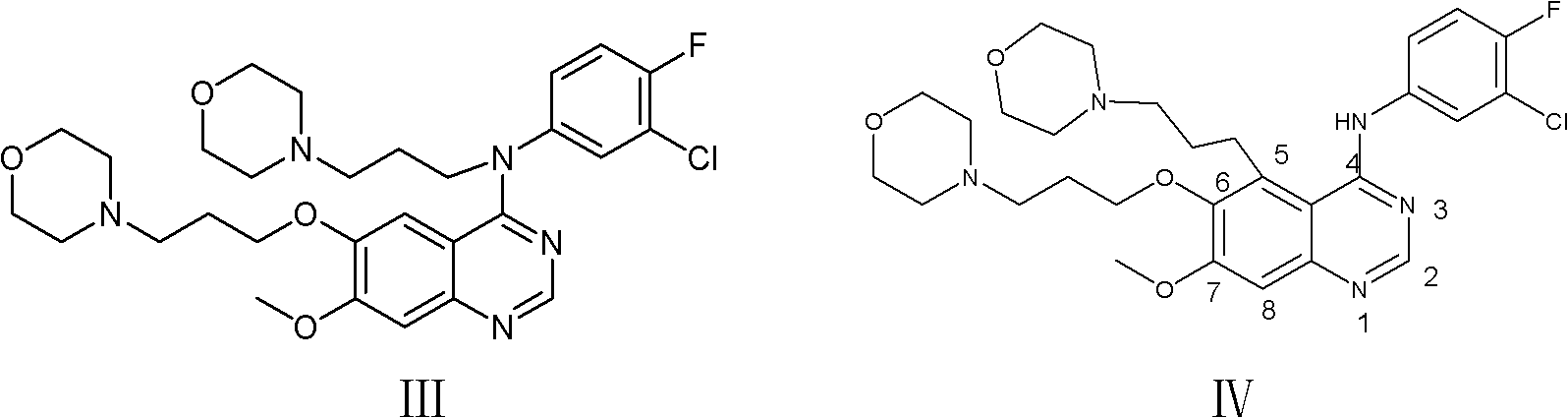
Preparation method of high-purity gefitinib
A technology of gefitinib and purity, applied in the field of preparation of gefitinib, can solve the problems of long reaction time and many impurities, and achieve the effects of simple operation, improved yield and reduced difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of gefitinib crude product: 40kg compound II 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-alcohol was added to 400L of N,N-dimethylformamide , add 35kg potassium carbonate (d(0.5) is 49μm), 4kg anhydrous magnesium sulfate, 25kg N-(3-chloropropyl)morpholine successively under stirring, react at 70°C, TLC detects that the reaction is complete after 5h, and cools down to room temperature After that, 2000L of purified water was added, centrifuged, and dried to obtain 47.6 kg of crude gefitinib, with a yield of 85.1% and a purity of 98.95%.
[0049] Refining of gefitinib: 47.6 kg of gefitinib obtained was added to 900 L of ethanol, refluxed for dissolution, cooled to -5 to 5° C., centrifuged, and dried to obtain 43.4 kg of gefitinib with a yield of 91.2%. The purity is 99.93%, and the largest single hetero compound IV is 0.04%.
Embodiment 2
[0051] Preparation of crude gefitinib: Add 40 g of 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-ol (compound II) to 400 ml of N,N-dimethylformaldehyde Add 40g of potassium carbonate (d(0.5) is 39μm), 10g of anhydrous sodium sulfate, and 25g of N-(3-chloropropyl)morpholine successively under stirring, and react at 85°C. After 4 hours, TLC detects that the reaction is complete. After reaching room temperature, 2L of purified water was added, suction filtered, and dried to obtain 48.2 g of crude gefitinib, with a yield of 86.2% and a purity of 98.87%.
[0052] Refining of Gefitinib: 48.2 g of Gefitinib obtained was added to 900 ml of ethanol, dissolved under reflux, cooled to -5 to 5° C., centrifuged, and dried to obtain 44.1 g of Gefitinib, with a yield of 91.5%. The purity is 99.92%, and the largest single hetero compound IV is 0.04%.
Embodiment 3
[0054] Preparation of crude gefitinib: Add 40 g of 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-ol (compound II) to 500 ml of N,N-dimethylformaldehyde Add 30g of potassium carbonate (d(0.5) is 39μm), 3g of anhydrous sodium sulfate, and 24g of N-(3-chloropropyl)morpholine successively under stirring, and react at 120°C. After 4 hours, TLC detects that the reaction is complete. After reaching room temperature, 2.5 L of purified water was added, suction filtered, and dried to obtain 47.3 g of crude gefitinib, with a yield of 84.6% and a purity of 99.02%.
[0055] Refining of Gefitinib: 47.3 g of Gefitinib obtained was added to 900 ml of ethanol, dissolved under reflux, cooled to -5 to 5° C., centrifuged, and dried to obtain 43.1 g of Gefitinib with a yield of 91.1%. The purity is 99.93%, and the largest single hetero compound IV is 0.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com