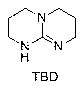
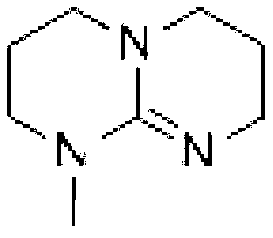
Preparation method of hexahydric dicycloguanidine based on guanidine hydrochloride
A technology of six-membered bicyclic guanidine and guanidine hydrochloride, which is applied in organic chemistry and other fields, can solve problems such as unreported MTBD, and achieve the effects of low price, easy industrial production, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: The synthesis equation is as follows.
[0022]
[0023] Step 1: Synthesis of TBD from Guanidine Hydrochloride:
[0024] Guanidine hydrochloride (95 g, 1 mol, 1 eq), 3,3-diaminodipropylamine (131 g, 1 mol, 1 eq) were added to the flask. Heat to 120°C to initiate the reaction, and continue to heat up to 150°C for 8 hours after initiation. Cool down to 70°C, add 300ml methanol, stir to dissolve. Then add 200ml of methanol solution of 1mol sodium methoxide to concentrate most of the methanol, add 300ml of dichloromethane, filter, and recrystallize the filter cake in 300ml of tetrahydrofuran, filter, wash the residue with 100ml of THF, and dry to obtain about 120g of TBD. The rate is 86%.
[0025] Step 2: Synthesis of MTBD from TBD:
[0026] Add TBD (50g, 1eq), potassium hydroxide (24g, 1.2eq) and 250ml of tetrahydrofuran to the bottle successively. Dimethyl sulfate (45.26g, 1eq) was added dropwise at room temperature. After dripping, react overnight at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com