Preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methyl propioric acid
A technology of trifluoromethyl and methylpropionic acid, which is applied in the field of preparation of 2-phenyl)-2-methylpropionic acid, which can solve the problems of high reaction risk factor, expensive auxiliary materials, and large production sewage discharge , to achieve the effect of short synthesis process, low raw material cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
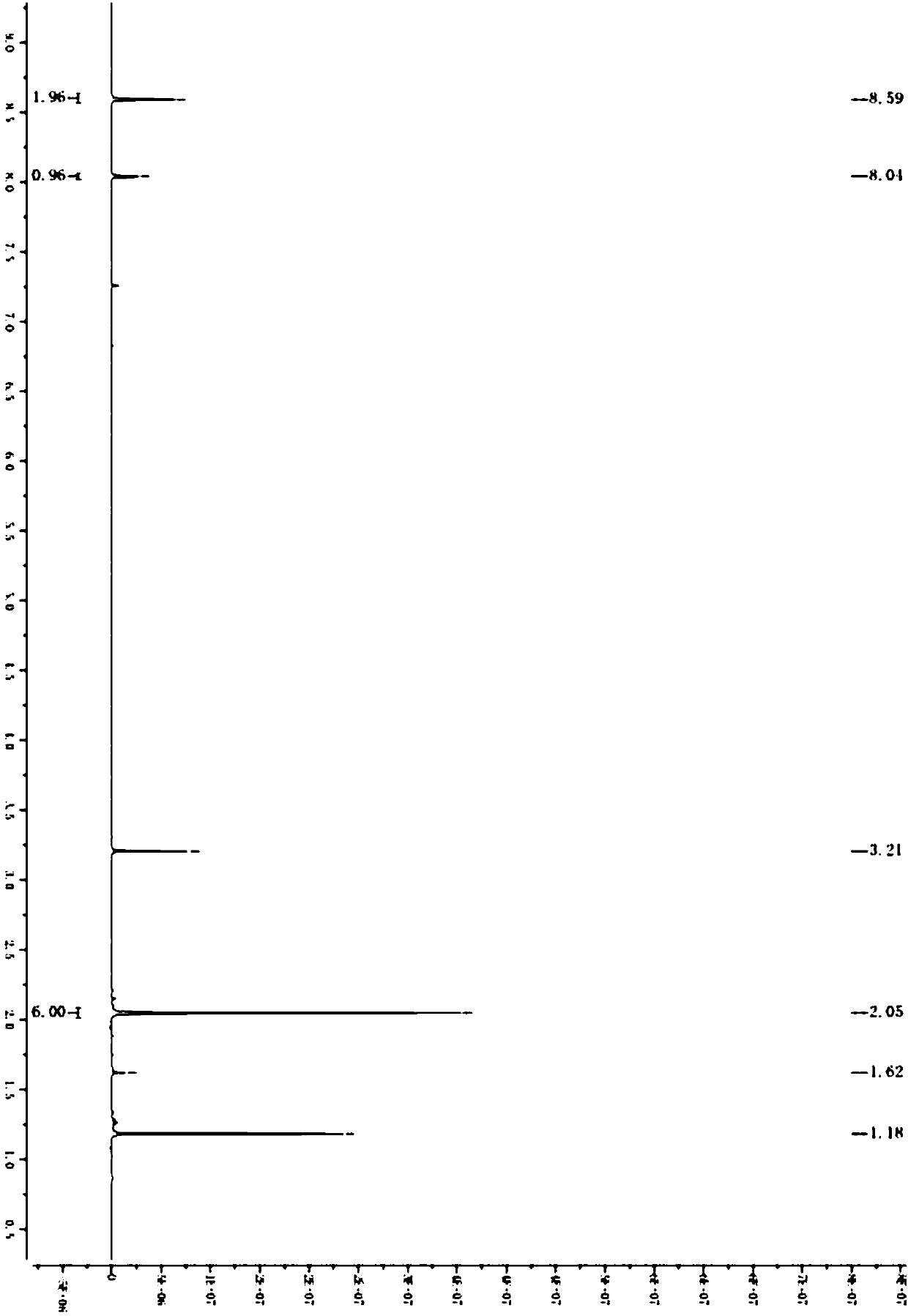
[0040] A kind of preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropionic acid, as shown in following chemical reaction formula:
[0041]
[0042] Wherein S1 prepares the specific parameters of 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropyl-1-one:
[0043]Isopropylmagnesium chloride (42g, 1.3eq) was added to a three-necked flask, and a solution of 3,5-bis(trifluoromethyl)bromobenzene (100g, 1.0eq) in tetrahydrofuran (300g) was added dropwise at 0°C. After the dropwise addition, the reaction system was reacted at 0°C for 1-1.5h, and then a solution of morpholine amide (43g, 0.8eq) in tetrahydrofuran (200 g) was added dropwise to the reaction system. Under reaction 3-4h. After the reaction, the reaction system was slowly added to water, the organic phase was separated, and the aqueous phase was extracted with methyl tert-butyl ether (500g,) again, the organic phase was concentrated to dryness, and column chromatography (PE:MTBE=30:1 ) to obtai...
Embodiment 2
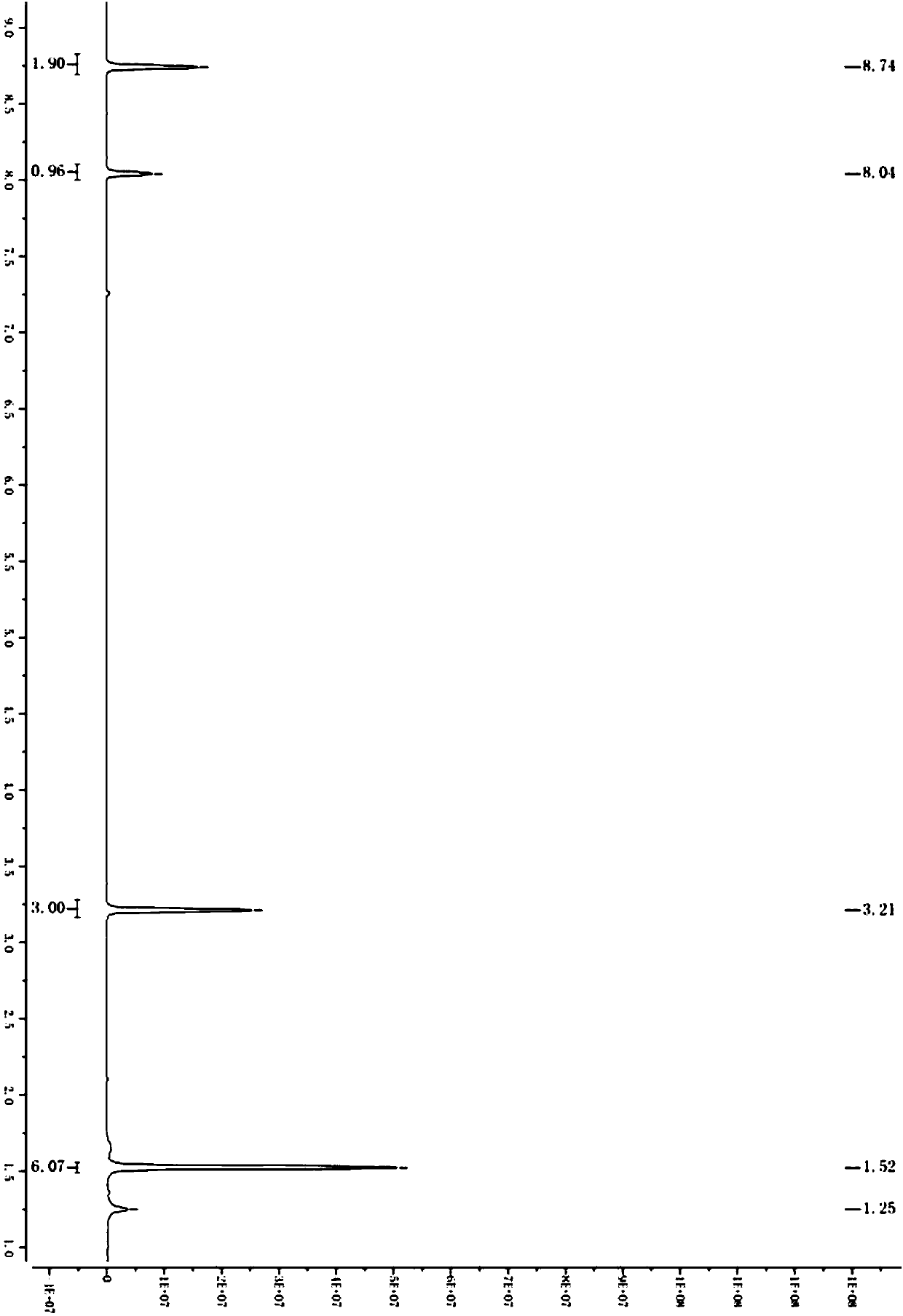
[0054] A kind of preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropionic acid, as shown in following chemical reaction formula:
[0055]
[0056] Wherein S1 prepares the specific parameters of 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropyl-1-one:
[0057] Isopropylmagnesium chloride (42g, 1.2eq) was added to a three-necked flask, and a solution of 3,5-bis(trifluoromethyl)bromobenzene (100g, 1.0eq) in tetrahydrofuran (300g) was added dropwise at 0°C. After the dropwise addition, the reaction system was reacted at 0°C for 1-1.5h, and then a solution of morpholine amide (43g, 0.8eq) in tetrahydrofuran (200 g) was added dropwise to the reaction system. Under reaction 3-4h. After the reaction, the reaction system was slowly added to water, the organic phase was separated, and the aqueous phase was extracted with methyl tert-butyl ether (500g,) again, the organic phase was concentrated to dryness, and column chromatography (PE:MTBE=30:1 ) to obta...
Embodiment 3
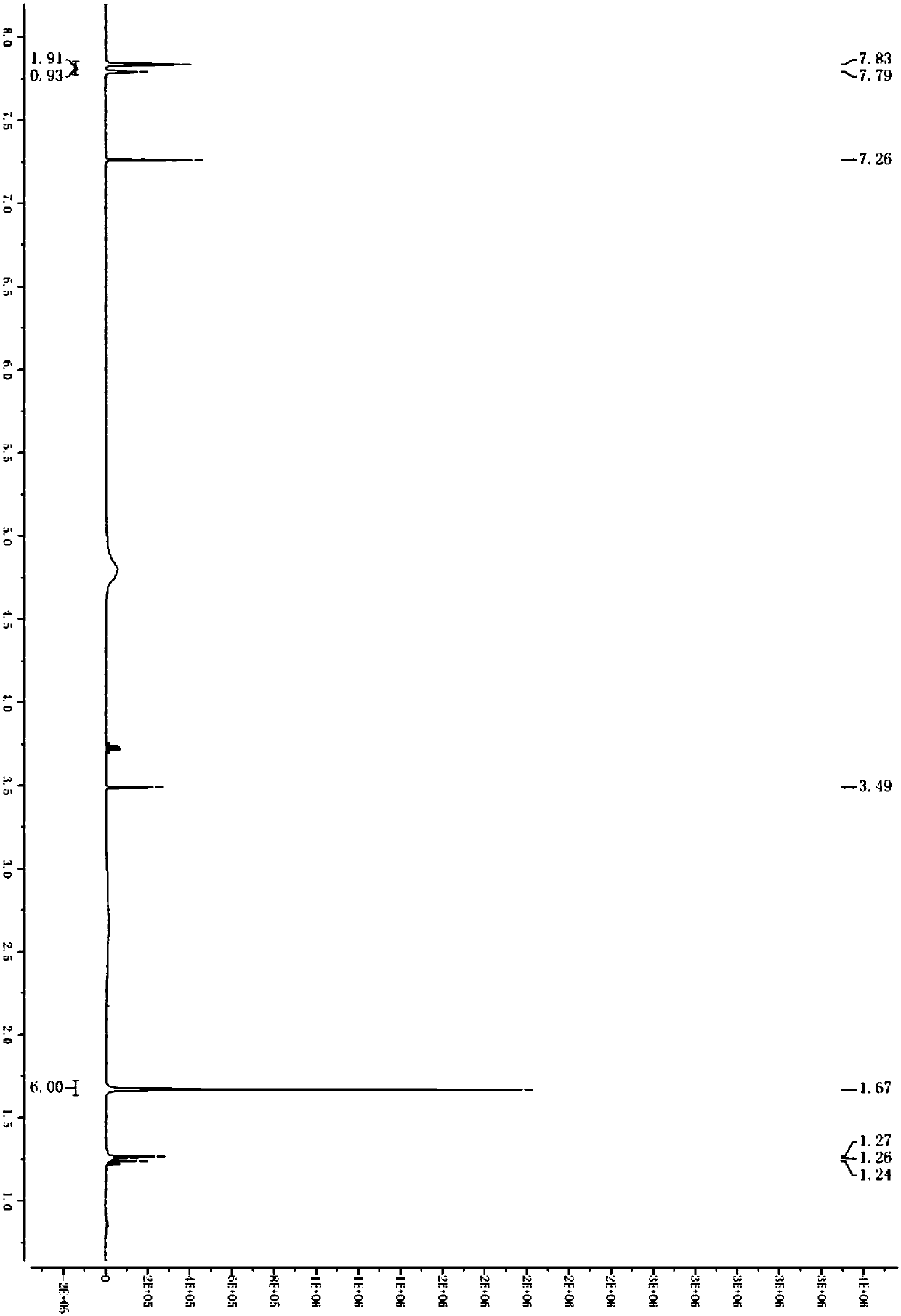
[0068] A kind of preparation method of synthetic 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropionic acid, as shown in following chemical reaction formula:
[0069]
[0070] Wherein S1 prepares the specific parameters of 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropyl-1-one:
[0071] Isopropylmagnesium chloride (42g, 1.4eq) was added to a three-necked flask, and a solution of 3,5-bis(trifluoromethyl)bromobenzene (100g, 1.0eq) in tetrahydrofuran (300g) was added dropwise at 0°C. After the dropwise addition, the reaction system was reacted at 0°C for 1-1.5h, and then a solution of morpholine amide (43g, 0.8eq) in tetrahydrofuran (200 g) was added dropwise to the reaction system. Under reaction 3-4h. After the reaction, the reaction system was slowly added to water, the organic phase was separated, and the aqueous phase was extracted with methyl tert-butyl ether (500g,) again, the organic phase was concentrated to dryness, and column chromatography (PE:MTBE=30:1 ) to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com