Preparation method of tetrabromobisphenol A bis(dibromoalkane)ether series compounds
A technology of dibromoalkyl and tetrabromobisphenol, which is applied in the field of compound preparation, can solve the problems of high energy consumption, long bromination reaction time, and low final product content, and achieve low reaction temperature, easy industrial production, and products The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
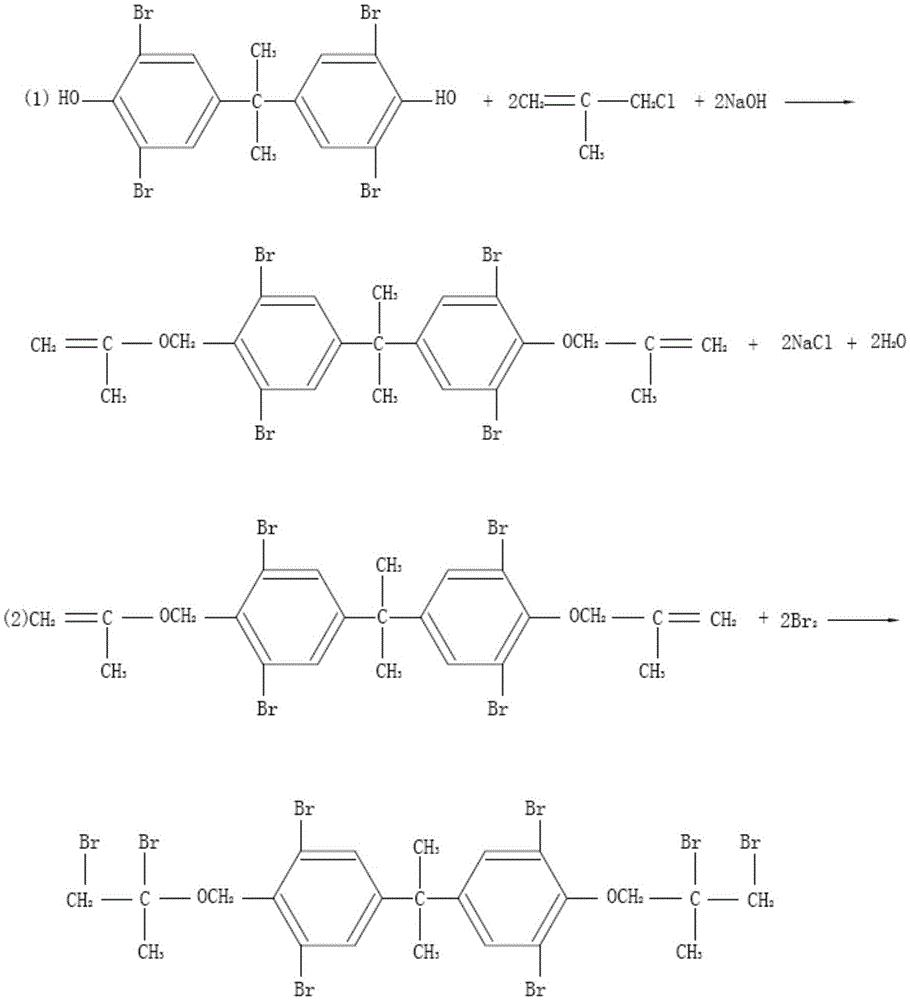
[0025] The preparation method of bis(2,3-dibromo-2-methylpropyl) ether of tetrabromobisphenol A, described preparation method comprises the following steps:
[0026] Take 1000kg of tetrabromobisphenol A, 2400kg of methanol, 2400kg of water, mix well, add 170kg of sodium hydroxide, stir to dissolve, adjust the pH to 8.0, then add 380kg of chlorinated isobutylene dropwise, and the chlorinated isobutylene will be dropped within 80 minutes. Reflux at 65°C for 6 hours. After the reaction, cool down to 25°C, centrifuge, take the filter cake, rinse with water, and dry at 75°C to obtain a white intermediate.
[0027] Take 700kg of intermediate, add 1700kg of dichloromethane, add 150kg of dioxane, stir to dissolve, pour 347kg of bromine into the metering tank, add bromine dropwise, and drop bromine completely in about 1 to 2 hours.
[0028] After dripping bromine, react at 42°C for 2 hours. After the reaction is completed, slowly add sodium sulfite aqueous solution (the weight ratio of...
Embodiment 2
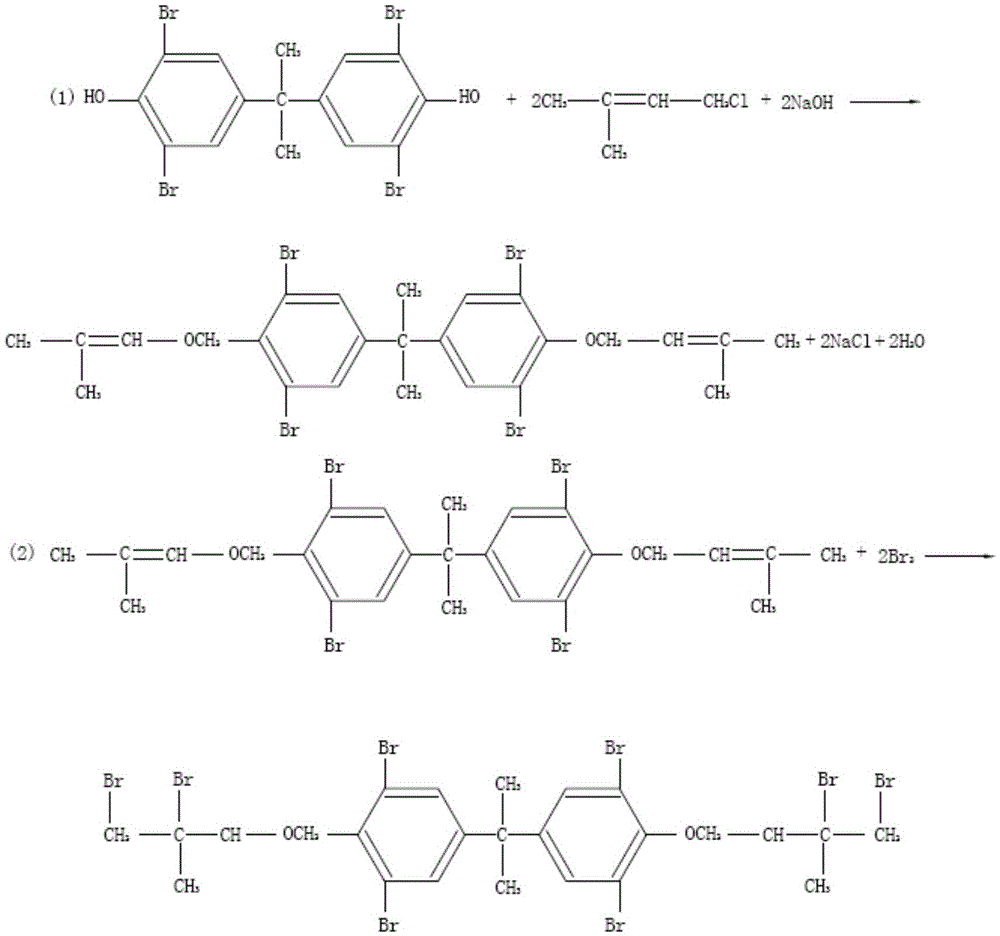
[0033] The preparation method of bis(3,4-dibromo-3-methylbutyl) ether of tetrabromobisphenol A, described preparation method comprises the following steps:
[0034] Take 1000kg of tetrabromobisphenol A, 2400kg of methanol, 2400kg of water, mix well, add 170kg of sodium hydroxide, stir to dissolve, adjust the pH to 8.5, then add 438kg of chlorinated isopentene dropwise. After dropping, the temperature was raised to 65°C and refluxed for 6 hours. After the reaction, the temperature was lowered to 25°C, centrifuged, the filter cake was taken, washed with water, and dried at 75°C to obtain a white intermediate.
[0035] Take 700kg of the intermediate, add 1700kg of dichloromethane, add 130kg of tetrahydrofuran, and 0.3kg of sodium bromide, stir to dissolve, put 330kg of bromine into the metering tank, add bromine dropwise, and drop the bromine completely in about 1 to 2 hours.
[0036] After dripping bromine, react at 44°C for 1 hour. After the reaction is completed, slowly add so...
Embodiment 3
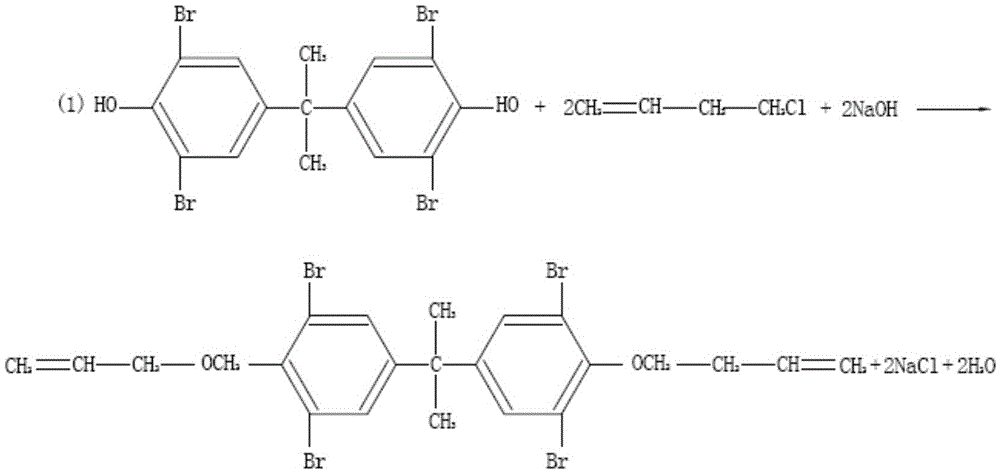
[0041] The preparation method of bis(3,4-dibromobutyl) ether of tetrabromobisphenol A, described preparation method comprises the following steps:
[0042] Take 1000kg of tetrabromobisphenol A, 2400kg of methanol, 2400kg of water, mix well, add 170kg of sodium hydroxide, stir to dissolve, adjust the pH to 9.5, then dropwise add 380kg of 4-chloro-1-butene, 4-chloro-1- The butene was dropped within 80 minutes, and the temperature was raised to 65°C to reflux for 6 hours. After the reaction, the temperature was lowered to 25°C, centrifuged, the filter cake was taken, rinsed with water, and dried at 75°C to obtain a white intermediate.
[0043] Take 700kg of the intermediate, add 1700kg of dichloromethane, add 130kg of tetrahydrofuran, and 0.3kg of sodium bromide, stir to dissolve, pour 347kg of bromine into the metering tank, add bromine dropwise, and drop the bromine completely in about 1 to 2 hours.
[0044] After dripping bromine, react at 36°C for 5 hours. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com