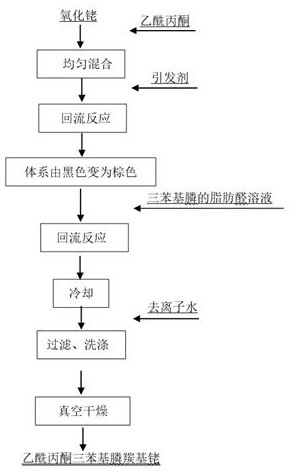
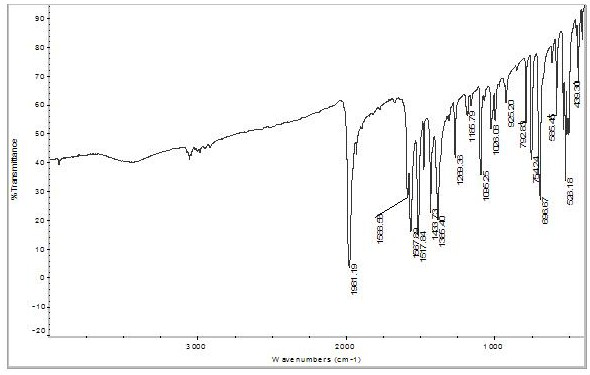
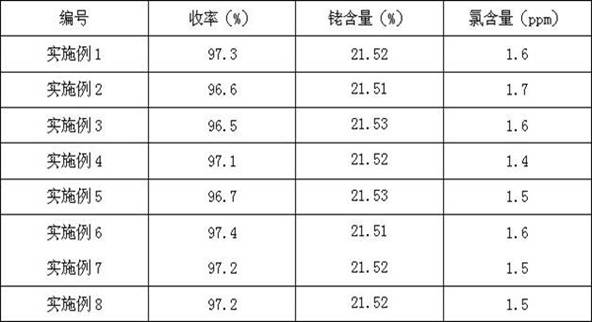
Preparation method of acetylacetone triphenylphosphine carbonyl rhodium
A technology of acetone triphenylphosphine and acetylacetone, which is applied in the field of precious metal catalyst synthesis, can solve the problems of harsh reaction conditions, olefin conversion rate decline, catalyst poisoning, etc., and achieve the goal of increasing product yield, improving product purity and reducing by-products the effect produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]Preparation method of acetylacetonone triphenylphosphinecarbonyl rhodium, comprising the following steps:
[0026] Step 1: In a three-mouth flask equipped with a reflux condenser, the rhodium content of 0.1g of rhodium oxide 0.122g is dissolved in 0.6g of acetylacetone solution, after stirring and mixing evenly, using a water ring vacuum pump, continuously evacuated under a vacuum degree of -0.08Mpa and injected nitrogen with a pressure of 0.02Mpa three times, the oxygen content in the three-mouth flask can be achieved less than 1000ppm;
[0027] Step 2, reflux: add 1ml of ammonia to the mixed solution prepared in step 1, heat up to 120 ° C, stir at the reflux temperature for 1.5h, when the solution is converted from black to brownish yellow, inject 0.28g triphenylphosphine and 0.05 g of anaerobic acetaldehyde, continue the reflux reaction at 120 °C, stir the reaction for 1.5 hours, stop heating, and cool the solution to room temperature;
[0028] Step 3, filtration washing:...
Embodiment 2
[0032] Preparation method of acetylacetonone triphenylphosphinecarbonyl rhodium, comprising the following steps:
[0033] Step 1, mixing: In the three-mouth flask equipped with a reflux condenser, the rhodium content of 0.1g of rhodium oxide 0.122g is dissolved in 1g of acetylacetonate solution, after stirring and mixing evenly, using a water ring vacuum pump, continuously evacuated under a vacuum degree of -0.08Mpa and injected nitrogen with a pressure of 0.02Mpa three times, the oxygen content in the three-mouth flask can be achieved less than 1000ppm;
[0034] Step 2, reflux: add 1ml of ammonia to the mixed solution prepared in step 1, heat up to 120 ° C, stir at the reflux temperature for 1.5h, when the solution is converted from black to brownish yellow, inject 0.31g triphenylphosphine and 0.08 g of anaerobic acetaldehyde at a time, continue the reflux reaction at 120 °C, stir the reaction for 1.5 hours, stop heating, and cool the solution to room temperature;
[0035]Step 3,...
Embodiment 3
[0039] Preparation method of acetylacetonone triphenylphosphinecarbonyl rhodium, comprising the following steps:
[0040] Step 1, mixing: In the three-mouth flask equipped with a reflux condenser, the rhodium content of 0.1g of rhodium oxide 0.122g is dissolved in 1g of acetylacetonate solution, after stirring and mixing evenly, using a water ring vacuum pump, continuously evacuated under a vacuum degree of -0.08Mpa and injected nitrogen with a pressure of 0.02Mpa three times, the oxygen content in the three-mouth flask can be achieved less than 1000ppm;
[0041] Step 2, reflux: add 1ml of ammonia to the mixed solution prepared in step 1, heat up to 120 ° C, stir at the reflux temperature for 1.5h, when the solution is converted from black to brownish yellow, inject 0.38g triphenylphosphine and 0.2 g of anaerobionaldehyde at a time, continue the reflux reaction at 120 °C, stir the reaction for 1.5 hours, stop heating, and cool the solution to room temperature;
[0042] Step 3, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com