Synthesis method of diclofenac sodium
A technology of diclofenac sodium and a synthesis method, applied in the field of diclofenac sodium synthesis, can solve the problems of unobtainable raw materials, three-waste pollution, and high cost, and achieve the effects of facilitating subsequent treatment, reducing the generation of by-products, and improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
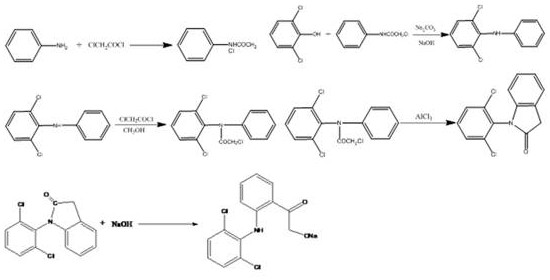
[0033] A kind of synthetic method of diclofenac sodium, comprises the following steps:
[0034](1) Put 2 parts of toluene, 0.5 parts of aniline and 0.005 parts of benzyltriethylammonium chloride into a dry four-necked bottle, start the tail gas absorption, stir and drop 0.6 parts of chloroacetyl chloride in the solution, drop The time is about 1.5h, the temperature is controlled at 15°C, and the temperature is slowly raised to 80°C after dropping, and a small amount of toluene is added after 4 hours of reflux and heat preservation, and the temperature is lowered to 60°C. Then add 1 part of 2,6-dichlorophenol and 0.6 part of sodium carbonate, heat up to 80°C, keep warm for 18h and then cool to 75°C. Add purified water, raise the temperature to 80°C, continue to stir and keep warm for 1h, let stand, extract the oil layer, add 0.4 parts of triethylamine, keep warm at 80°C for 6h. Wash with water, separate the oil layer, distill under normal pressure and then under reduced pressu...
Embodiment 2
[0039] (1) Put 8 parts of toluene, 0.8 parts of aniline and 0.5 parts of tetrabutylammonium bromide into a dry four-necked bottle, start the tail gas absorption, stir and drop 1.0 parts of chloroacetyl chloride in the solution, and the dropping time is About 1.5h, the temperature is controlled at 25°C, and the temperature is slowly raised to 100°C after dropping, and a small amount of toluene is added after reflux for 4 hours, and the temperature is lowered to 70°C. Then add 1 part of 2,6-dichlorophenol and 0.6 part of sodium carbonate, raise the temperature to 120°C, keep it warm for 18 hours and then cool to 75°C. Add purified water, raise the temperature to 100°C, continue to stir and keep warm for 1h, let it stand still, extract the oil layer, add 2.0 parts of sodium methoxide, raise the temperature to 110°C and keep warm for 6h. Wash with water, separate the oil layer, distill under normal pressure and then under reduced pressure, recover toluene, and collect fractions at...
Embodiment 3
[0044] (1) Put 5 parts of toluene, 0.6 parts of aniline and 0.25 parts of catalyst into a dry four-necked bottle. The catalysts are tetrabutylammonium chloride and tetrabutylammonium bisulfate. Turn on the tail gas absorption, stir and drop in the solution Add 0.8 parts of chloroacetyl chloride for about 1.5 hours, control the temperature at 20°C, slowly raise the temperature to 90°C after dropping, add a small amount of toluene after reflux for 4 hours, and cool down to 65°C. Then add 1 part of 2,6-dichlorophenol and 0.6 part of sodium carbonate, heat up to 100°C, keep warm for 18h and then cool to 75°C. Add purified water, raise the temperature to 90°C, continue to stir and keep warm for 1h, let stand, extract the oil layer, add 1.2 parts of sodium ethoxide and tert-butanol, keep warm at 95°C for 6h. Wash with water, separate the oil layer, distill under normal pressure and then under reduced pressure, recover toluene, and collect fractions at 180-260°C through reduced-press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com