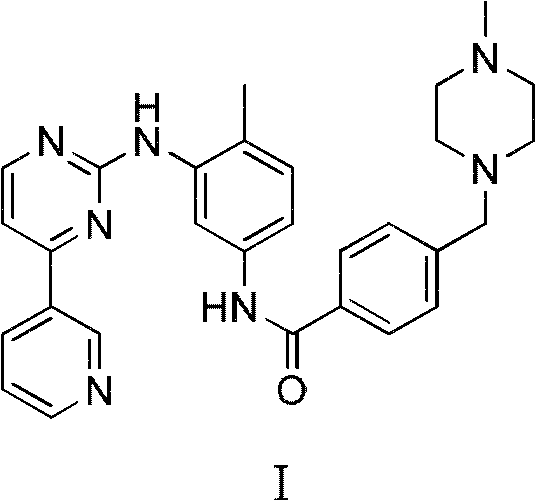
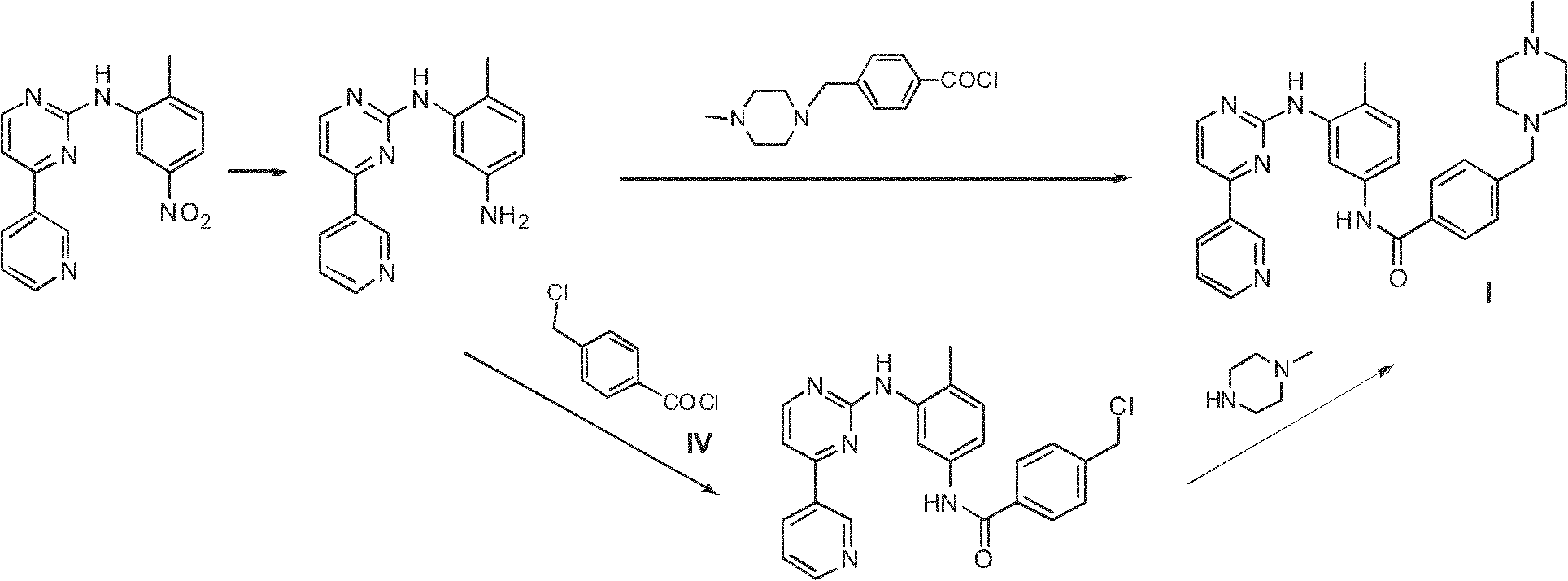
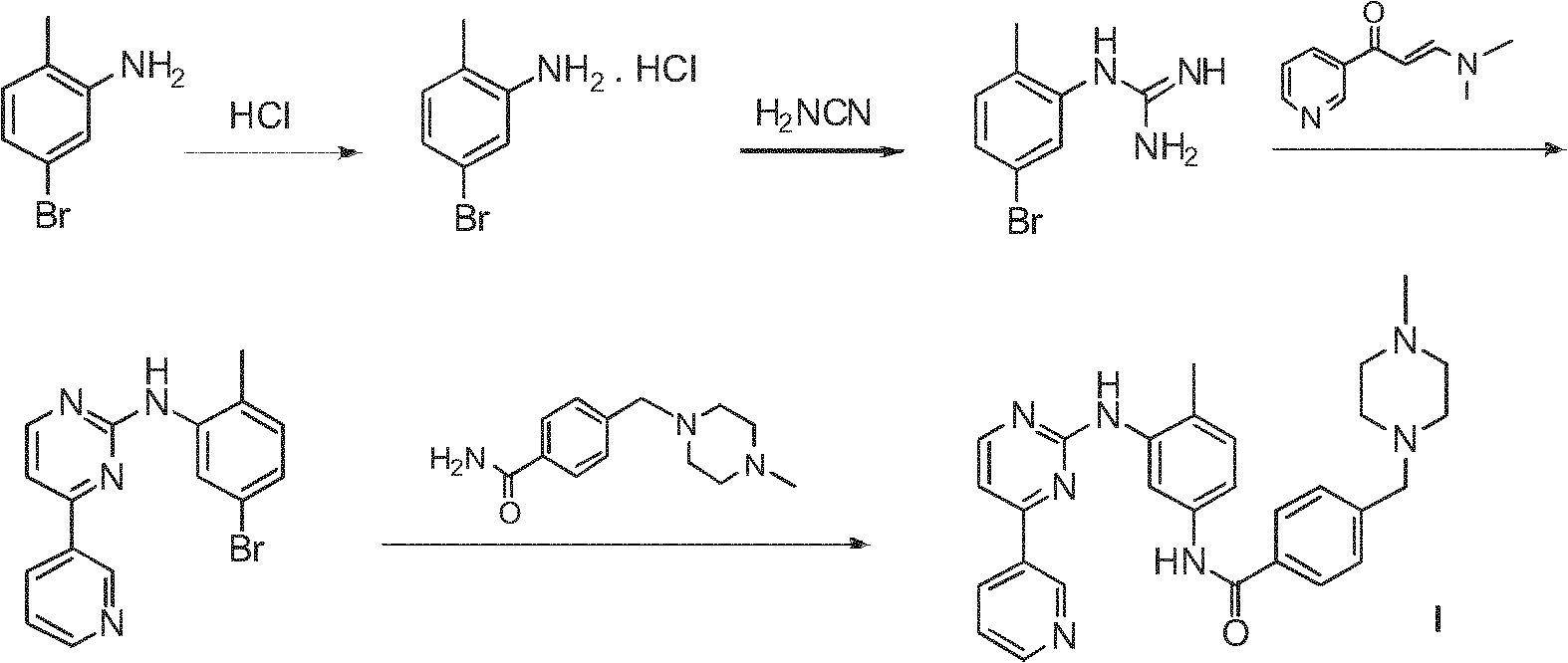
Method for preparing anti-tumor medicine imatinib
An anti-tumor drug and reaction process technology, applied in the field of synthesis of anti-tumor drug imatinib, can solve the problems of low yield, increased operation, complicated preparation, etc., and achieve easy large-scale production, meet production requirements, The effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of III:
[0048] Under a nitrogen atmosphere, 50ml of anhydrous dimethyl sulfoxide was added to the reaction flask, and 4-chloromethylbenzoyl chloride (22.7g, 0.12mol) and triethylamine (30.0ml, 0.20mol) were added successively under stirring. . Under ice-bath conditions, slowly add dropwise a mixed solution of 4-methyl-3-bromoaniline (18.6g, 0.10mol) and 50ml of anhydrous dimethyl sulfoxide, and control the temperature of the system at 0 to 10 °C range. After the addition was complete, the reaction was continued at room temperature for 10 hours, followed by TLC until the 4-methyl-3-bromoaniline spot disappeared. N-methylpiperazine (15.5ml, 0.14mol) was added dropwise, and the reaction solution was continued to react for 4 hours after addition, followed by TLC until the intermediate spot disappeared. Stop the reaction, pour into 200ml of deionized water, stir, filter with suction, wash the filter cake with appropriate amount of water to obtain 35.1g of wh...
Embodiment 2
[0053] Preparation of III:
[0054] Under a nitrogen atmosphere, 50ml of anhydrous N,N-dimethylformamide was added to the reaction flask, and 4-chloromethylbenzoyl chloride (22.7g, 0.12mol), anhydrous sodium carbonate (21.2 g, 0.20mol). Under ice-bath conditions, slowly add dropwise a mixed solution of 4-methyl-3-bromoaniline (18.6g, 0.10mol) and 50ml of anhydrous N,N-dimethylformamide, and control the temperature of the system during the dropwise addition In the range of 0 ~ 10 ℃. After the addition, the reaction was continued at room temperature for 10 hours, TLC followed the reaction until the 4-methyl-3-bromoaniline spots disappeared, N-methylpiperazine (15.5ml, 0.14mol) was added dropwise, and the reaction was continued for 4 Hours, TLC tracking until the intermediate spot disappears. Stop the reaction, pour into 200ml of deionized water, stir, suction filter, and wash the filter cake with appropriate amount of water to obtain 32.6g of earth-colored powdery solid III, ...
Embodiment 3
[0056] Preparation of I
[0057] Under a nitrogen atmosphere, dissolve compound III (20.1g, 0.05mol) and compound II (10.3g, 0.06mol) in 200ml of anhydrous N, N-dimethylformamide (DMF), and add them in turn under stirring at room temperature Potassium carbonate solid (20.7g, 0.15mol), cuprous iodide (3.8g, 0.02mol) and N, N'-dimethylethylenediamine (2.1ml, 0.02mol), heated to reflux for 20 hours, TLC Track until the spot of raw material III disappears; add 50ml concentrated ammonia water, 50ml saturated sodium chloride solution, 100ml ethyl acetate, separate the organic phase, extract the aqueous phase with ethyl acetate (3×50ml), combine the organic phases, and saturated chlorinate Wash with sodium (3×50ml), dry over anhydrous sodium sulfate, filter with suction, and the residue after the filtrate is evaporated to dryness under reduced pressure is a light yellow solid, which is recrystallized from acetonitrile to obtain 19.0 g of white needle-like crystals I; mp 202 ~206℃, y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com