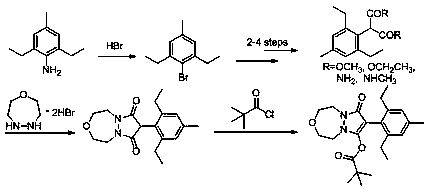
Synthesis method of pinoxaden
A technology of pinoxaden and its synthetic method, which is applied in the field of pinoxaden synthesis, can solve the problems of high cost, long reaction steps, cumbersome operation, etc., and achieve the effects of simple operation, less environmental pollution, and high economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1), the synthesis of 2,6-diethyl-4-methylbromobenzene
[0030] Hydrogen bromide adds in twice in the following steps,
[0031] First in an ice bath, dissolve 2,6-diethyl-4-methylaniline (12 g, 73.5 mmol) in hydrobromic acid (40%, 14.9 mL, first addition), and ethanol (90 mL) Add them together into the reaction flask, and then add a solution of sodium nitrite (5.58 g, 80.9 mmol) in water (6 mL) dropwise to the solution obtained above. Then the above solution was added to the solution of cuprous bromide (2.11g, 14.7mmol) in hydrobromic acid (40%, 68.9mL, added for the second time), and the temperature was raised to 80°C, and the reaction was kept for 3h, and the reaction was detected by TLC. . Then the reaction solution was cooled to room temperature, diluted with water (50 mL), extracted three times with ethyl acetate (100 mL), the organic layer was collected, washed once with sodium bicarbonate solution, dried over magnesium sulfate, and the solvent was distilled off...
Embodiment 2
[0039] (1), the synthesis of 2,6-diethyl-4-methylbromobenzene
[0040] In an ice bath, 2,6-diethyl-4-methylaniline (12g, 73.5mmol) was dissolved in hydrobromic acid (40%, 24.1mL), and ethanol (90mL) was added to the reaction flask together, A solution of sodium nitrite (6.8 g, 98.5 mmol) in water (6 mL) was then added dropwise to the above obtained solution. Then the solution obtained above was added to a solution of cuprous bromide (6.2g, 44.1mmol) in hydrobromic acid (40%, 121.3mL), the temperature was raised to 95°C, and the reaction was incubated for 2 hours. TLC detected that the reaction was complete. Then the reaction solution was cooled to room temperature, diluted with water (50 mL), extracted three times with ethyl acetate (100 mL), the organic layer was collected, washed once with sodium bicarbonate solution, dried over magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chr...
Embodiment 3
[0048] (1), the synthesis of 2,6-diethyl-4-methylbromobenzene
[0049] In an ice bath, 2,6-diethyl-4-methylaniline (12g, 73.5mmol) was dissolved in hydrobromic acid (40%, 27.9mL), and ethanol (90mL) was added to the reaction flask together, A solution of sodium nitrite (7.61 g, 110.25 mmol) in water (6 mL) was then added dropwise to the above obtained solution. Then the solution obtained above was added to a solution of cuprous bromide (8.4g, 58.8mmol) in hydrobromic acid (40%, 140.7mL), heated to 80°C, kept for 2 hours, and TLC detected that the reaction was complete. Then the reaction solution was cooled to room temperature, diluted with water (50 mL), extracted three times with ethyl acetate (100 mL), the organic layer was collected, washed once with sodium bicarbonate solution, dried over magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (petroleum ether) to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com