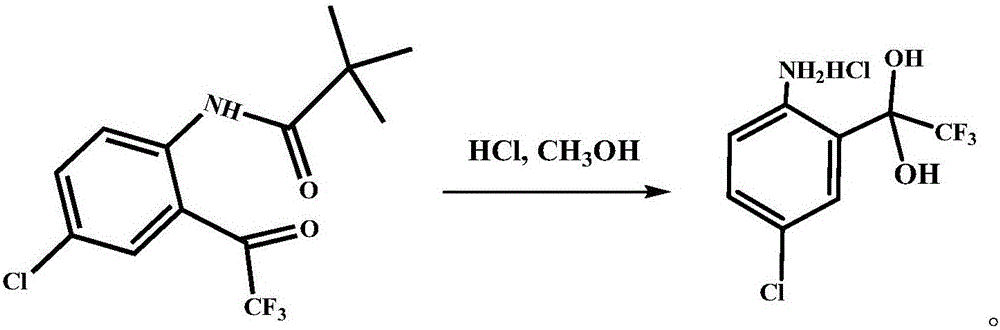
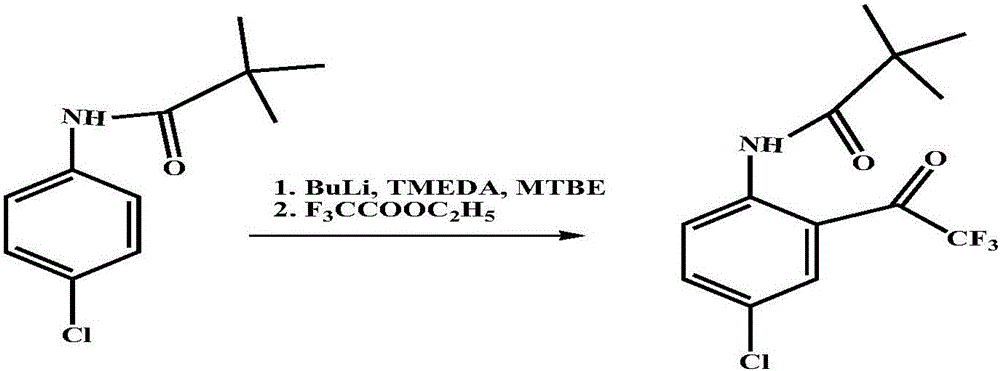Preparation method of 4-chloro-2-(trifluoroacetyl) aniline hydrochloride hydrate
A technology of trifluoroacetylphenyl and trifluoroacetyl, which is applied in the field of compound preparation, can solve the problems of difficult treatment and high cost, and achieve the effects of reducing the emission of "three wastes", low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
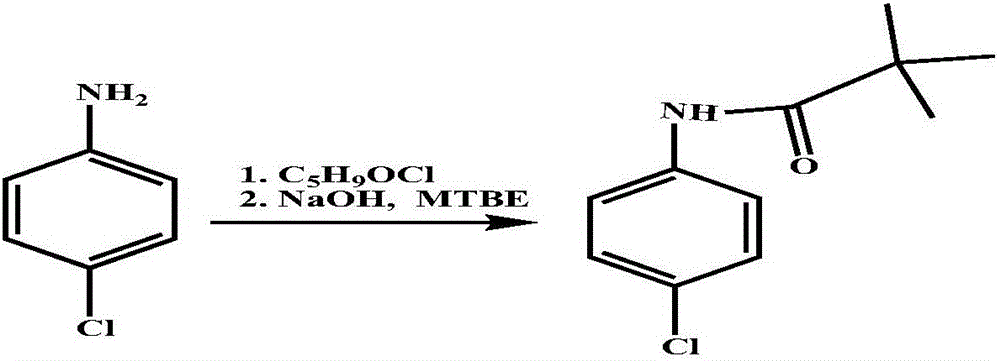
[0018] ① React p-chloroaniline with pivaloyl chloride to obtain 4-chloro-N-pivaloyl anilide: put 40.2g p-chloroaniline and 250mL toluene into the reactor, cool down to 0-5°C, add 30% sodium hydroxide Solution 45.4g, control the temperature at 5-15°C, add 41.6g of pivaloyl chloride dropwise, drop over 1 hour, then keep warm at 5-15°C for 1 hour, separate layers, wash the organic layer twice, cool to -5°C, Keep warm for 2 hours, filter with suction, wash the filter cake with water, drain it, and dry the filter cake under vacuum at 70°C for 24 hours to obtain 64.5 g of 4-chloro-N-pivalanilide with a content of 99.4% and a yield of 96.8%.
[0019] ② 4-Chloro-N-pivalanilide reacts with ethyl trifluoroacetate under the action of butyllithium to obtain N-(4-chloro-2 trifluoroacetylphenyl)-pivalanamide: in the reactor Add 52.9g of 4-chloro-N-pivalanilide, 500mL of methyl tert-butyl ether, 29g of tetramethylethylenediamine, stir, cool to -20°C, and dropwise add butyllithium hexane with...
Embodiment 2
[0022] ① React p-chloroaniline with pivaloyl chloride to obtain 4-chloro-N-pivaloyl anilide: put 40.2g p-chloroaniline and 250mL toluene into the reactor, cool down to 0-5°C, add 30% sodium hydroxide Solution 45.4g, control the temperature at 5-15°C, add 41.6g of pivaloyl chloride dropwise, drop over 1 hour, then keep warm at 5-15°C for 1 hour, separate layers, wash the organic layer twice, cool to -5°C, Keep warm for 2 hours, filter with suction, wash the filter cake with water, drain it, and dry the filter cake under vacuum at 70°C for 24 hours to obtain 51.7 g of 4-chloro-N-pivalanilide, with a content of 99.4% and a yield of 96.8%.
[0023] ② 4-Chloro-N-pivalanilide reacts with ethyl trifluoroacetate under the action of butyllithium to obtain N-(4-chloro-2 trifluoroacetylphenyl)-pivalanamide: in the reactor Add 52.9g of 4-chloro-N-pivalanilide, 500mL of methyl tert-butyl ether, 29g of tetramethylethylenediamine, stir, cool to -20°C, and dropwise add butyllithium hexane wit...
Embodiment 3
[0026] ① React p-chloroaniline with pivaloyl chloride to obtain 4-chloro-N-pivaloyl anilide: put 40.2g p-chloroaniline and 250mL toluene into the reactor, cool down to 0-5°C, add 30% sodium hydroxide Solution 45.4g, control the temperature at 5-15°C, add 41.6g of pivaloyl chloride dropwise, drop over 1 hour, then keep warm at 5-15°C for 1 hour, separate layers, wash the organic layer twice, cool to -5°C, Keep warm for 2 hours, filter with suction, wash the filter cake with water, drain it, and dry the filter cake under vacuum at 70°C for 24 hours to obtain 51.7 g of 4-chloro-N-pivalanilide, with a content of 99.4% and a yield of 96.8%.
[0027] ② 4-Chloro-N-pivalanilide reacts with ethyl trifluoroacetate under the action of butyllithium to obtain N-(4-chloro-2 trifluoroacetylphenyl)-pivalanamide: in the reactor Add 52.9g of 4-chloro-N-pivalanilide, 500mL of methyl tert-butyl ether, 29g of tetramethylethylenediamine, stir, cool to -20°C, and dropwise add butyllithium hexane wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com