Process for preparing chloro-pivalyl chloride
A technology for chloropivaloyl chloride and crude chloropivaloyl chloride, which is applied in carboxylate preparation, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as non-industrial production methods, and achieve low cost and easy The effect of control and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Industrial example: an example of producing 1 ton of chlorpivaloyl chloride with a content above 99.5%.
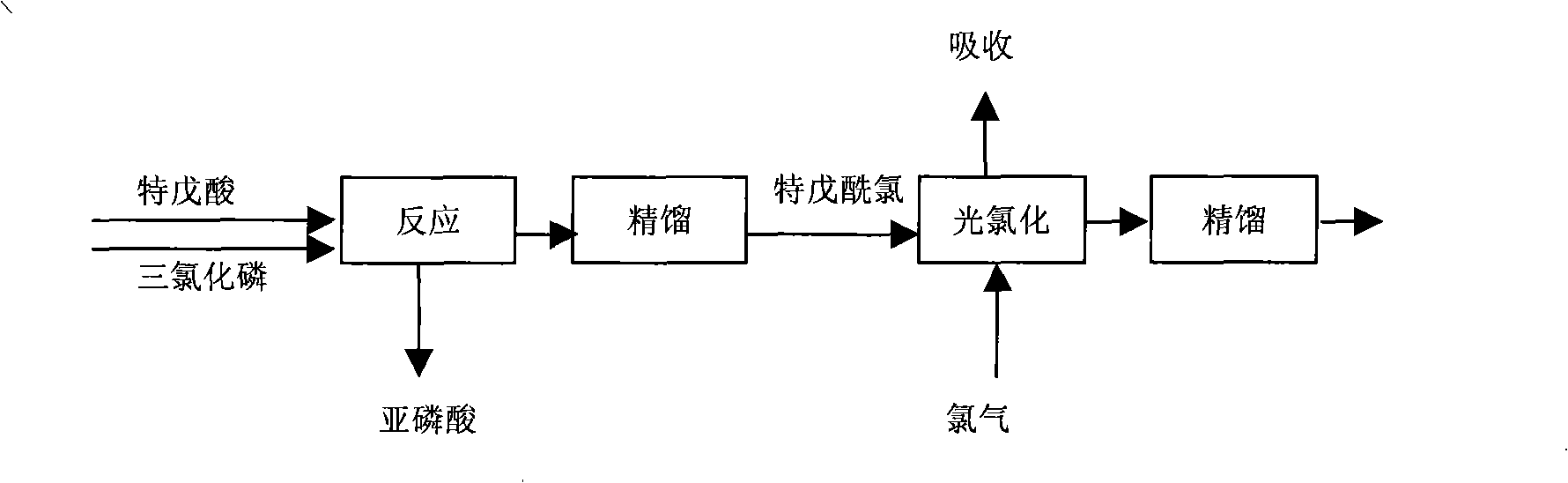
[0028] 1, in reaction step (1), the pivaloyl chloride (claiming trimethylacetyl chloride again, structural formula is (CH 3 ) 3 CCOCl) molecular formula is C 5 h 9 ClO, molecular weight 120.5)
[0029] Raw materials used (1) pivalic acid, content > 98% (2) phosphorus trichloride, content 98%
[0030] at 2m 3 Drop into 937kg pivalic acid and 405kg phosphorus trichloride in the reactor. Slowly raise the temperature under stirring for 2 hours, rise to 55 ° C, continue to keep warm and stir for 2 hours, let stand to separate the upper layer clear night, rectify, collect the fraction at 104-105 ° C, and obtain 1050 kg of fine pivaloyl chloride, with a content of 99.5%. The rate is above 92%.
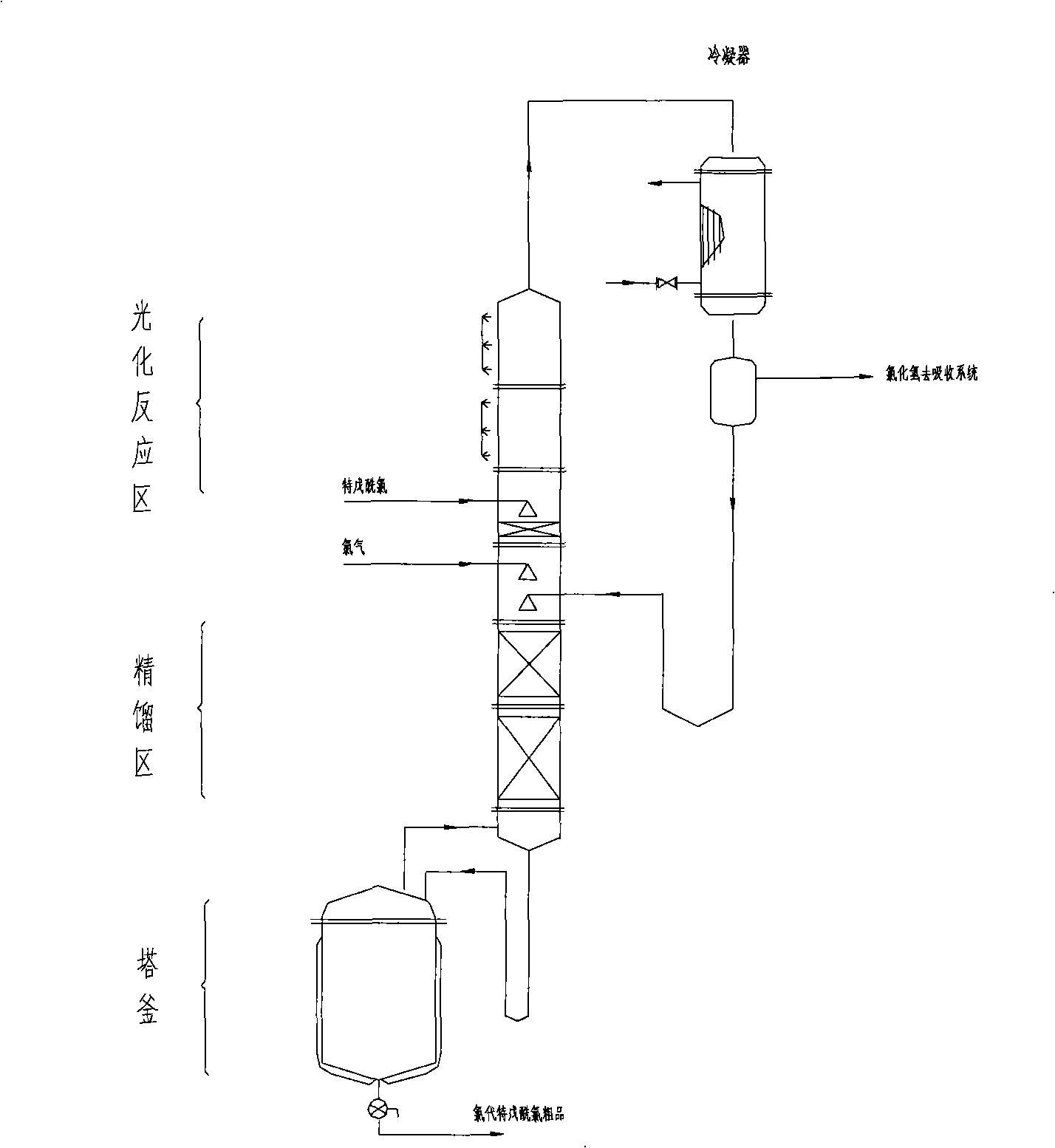
[0031] 2. In the reaction step (2), the final product 3-chloro-2,2-dimethylpropionyl chloride (also known as chloropivaloyl chloride, structural formula ClCH 2 (CH 3 ) 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com