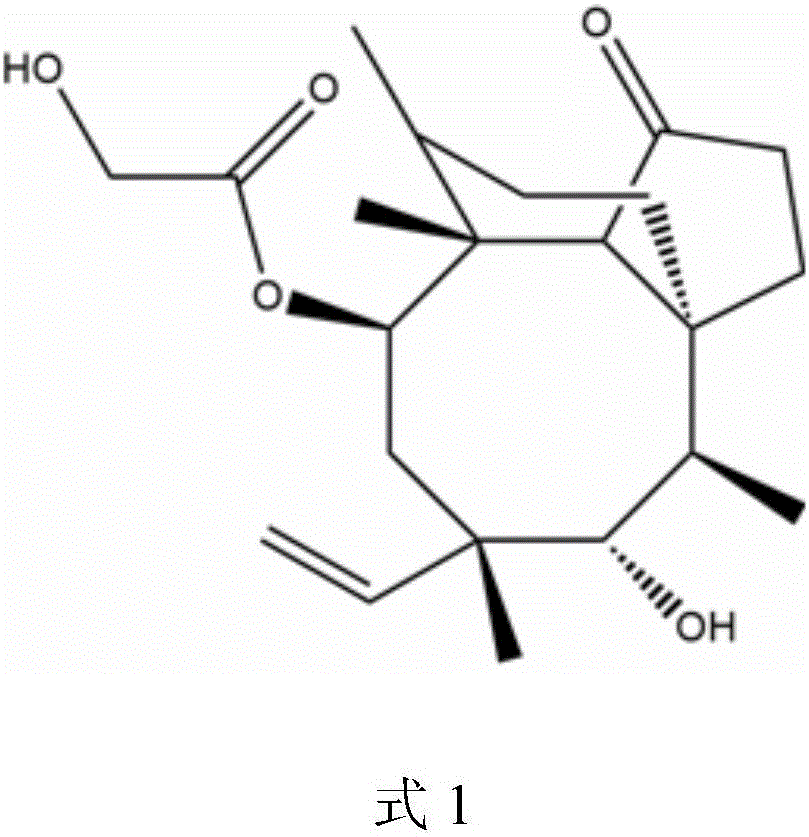
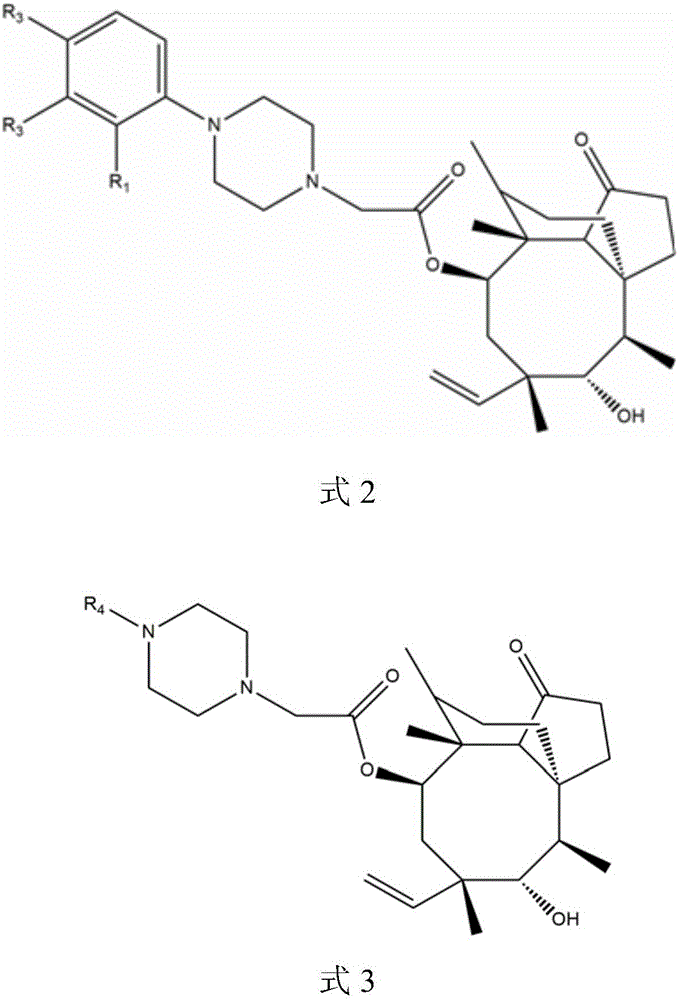
Pleuromutilin derivatives with piperazine side chain, and preparation method and application thereof
A technology for pleuromutilin and derivatives, which is applied in the field of pleuromutilin derivatives and its preparation, can solve the problem of rare drug-resistant bacteria, achieve good in vitro antibacterial activity and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of 14-O-[4-(2-oxomethylphenyl)piperazin-1-yl]acetyl muulin (compound 1)
[0048]0.481g (0.905mmol) of intermediate I was dissolved in 10ml of acetonitrile, 0.203g (1.36mmol) of sodium iodide and 0.125g (0.905mmol) of potassium carbonate were added, and heated to reflux for 1 hour. Dissolve 0.192g (1.00mmol) of 1-(2-oxomethylphenyl)piperazine in 10ml of acetonitrile, slowly add to the above mixed solution and continue to heat and reflux for 2h, transfer the reaction solution to a separatory funnel after reaction, and add 3. Take 50ml of water, 50ml of chloroform, shake, let stand to separate layers, take the organic phase, dehydrate it with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain the crude product as a solid. The solid was dissolved with 5 ml of ethyl acetate, and thoroughly mixed with 1 g of 200-300 mesh silica gel powder for chromatography. After the natural evaporation of ethyl acetate, the above...
Embodiment 2
[0049] Example 2: Synthesis of 14-O-[4-(3-oxomethylphenyl)piperazin-1-yl]acetyl muulin (compound 2)
[0050] 0.481g (0.905mmol) of intermediate I was dissolved in 10ml of acetonitrile, 0.203g (1.36mmol) of sodium iodide and 0.125g (0.905mmol) of potassium carbonate were added, and heated to reflux for 1 hour. Dissolve 0.192g (1.00mmol) of 1-(3-oxymethylphenyl)piperazine in 10ml of acetonitrile, slowly add to the above mixed solution and continue to heat and reflux for 2h, transfer the reaction solution to a separatory funnel after reaction, and add Mix 50ml of water and 50ml of chloroform, shake, let stand to separate layers, take the organic phase, dehydrate it with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain the crude product as a solid. The solid was dissolved with 5 ml of ethyl acetate, and thoroughly mixed with 1 g of 200-300 mesh silica gel powder for chromatography. After the natural evaporation of ethyl acetate, the above...
Embodiment 3
[0051] Example 3: Synthesis of 14-O-[4-(4-oxomethylphenyl)piperazin-1-yl]acetyl muulin (compound 3)
[0052] 0.481g (0.905mmol) of intermediate I was dissolved in 10ml of acetonitrile, 0.203g (1.36mmol) of sodium iodide and 0.125g (0.905mmol) of potassium carbonate were added, and heated to reflux for 1 hour. Dissolve 0.192g (1.00mmol) of 1-(4-oxymethylphenyl)piperazine in 10ml of acetonitrile, slowly add to the above mixture and continue to heat and reflux for 2h, transfer the reaction solution to a separatory funnel after reaction, and add Mix 50ml of water and 50ml of chloroform, shake, let stand to separate layers, take the organic phase, dehydrate it with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain the crude product as a solid. The solid was dissolved with 5 ml of ethyl acetate, and thoroughly mixed with 1 g of 200-300 mesh silica gel powder for chromatography. After the natural evaporation of ethyl acetate, the above crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com