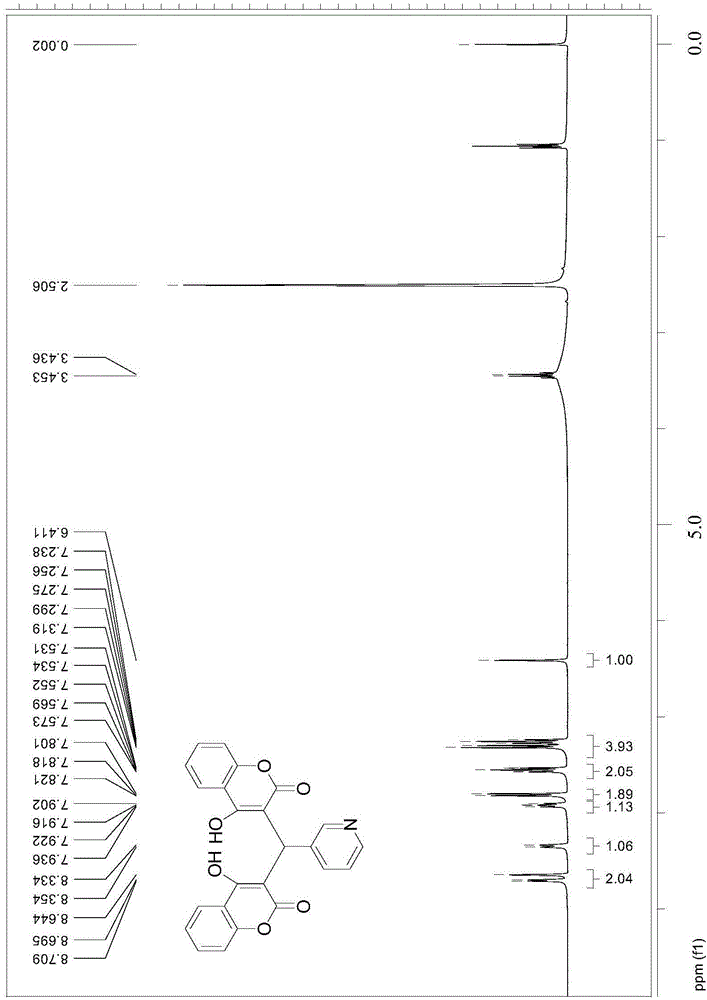
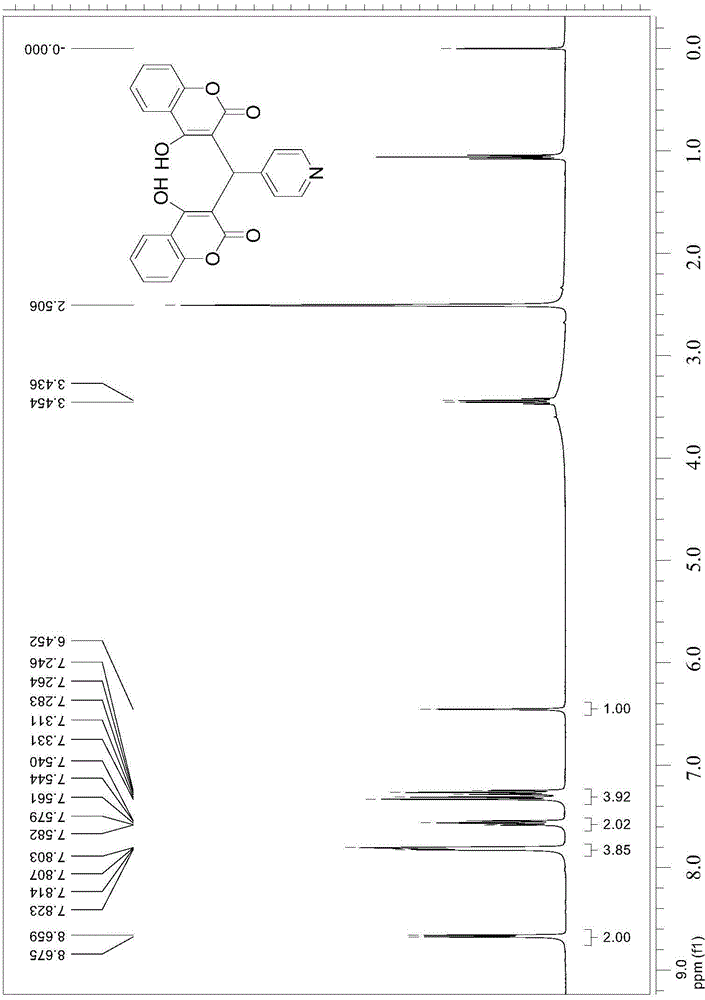
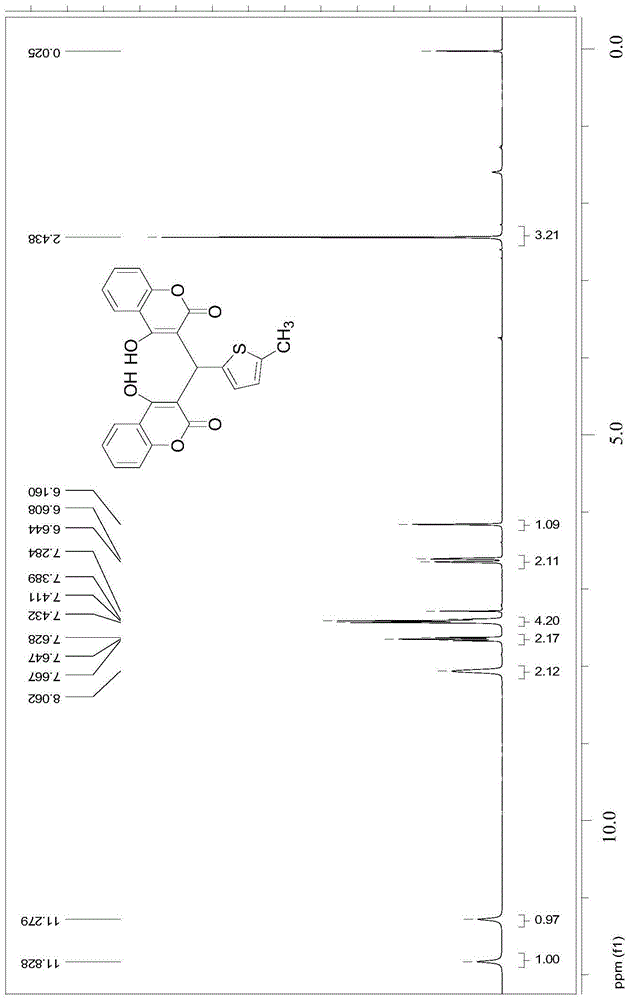
Thenylidene bishydroxycoumarin compound and application thereof
A technology of thiophene methylene dicoumarins and compounds, which is applied in the field of dicoumarins and their application in sterilization, and can solve toxic effects, no antibacterial activity, toxicity of coumarins, etc. problem, to achieve strong antibacterial effect and good antibacterial activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The bacteriostasis test of compound in 9 that embodiment 1 synthesizes is as follows:
[0065] 1 material
[0066] 1.1 Experimental strains
[0067] as table 1 As shown, 2 strains of G + strains (S. aureus, S. epidermidis) and 3 strains of G - Bacterial strains (Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli) were used in this part of the experiment, and the above-mentioned bacterial strains were all obtained from nation Provided by the Institute for the Control of Pharmaceutical and Biological Products of the Ministry of Health.
[0068] Table 1
[0069]
[0070]
[0071] 1.2 Main solution formula
[0072] 1.2.1 M-H broth medium
[0073] 2.5g M-H broth dry powder, add 125μl MgCl 2 solution (with Mg 2+ 10mg / ml) and 200μl CaCl 2 solution (with Ca 2+ 10mg / ml), then dilute to 100ml with deionized water, and autoclave.
[0074] 1.2.2 M-H agar medium
[0075] 3. 8g M-H agar medium dry powder, add 100ml distilled water, mix well, let i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com