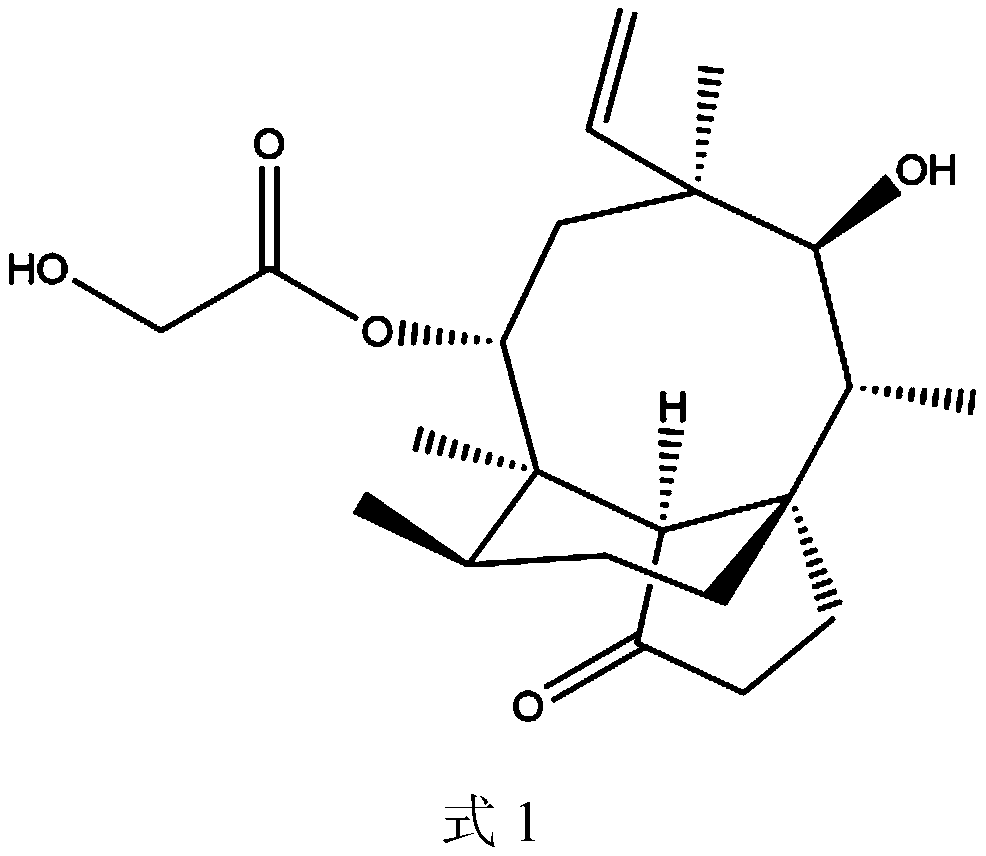
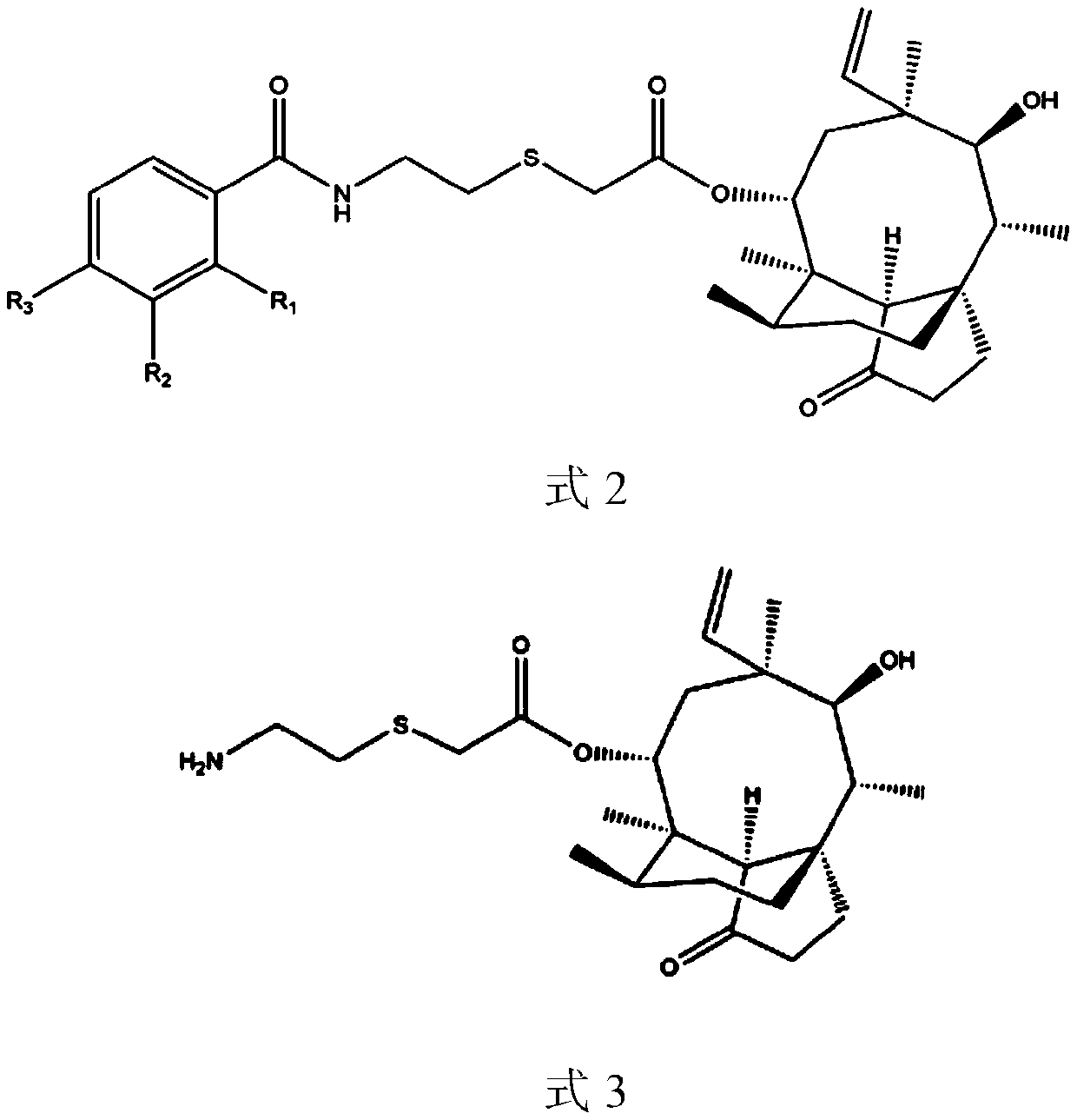
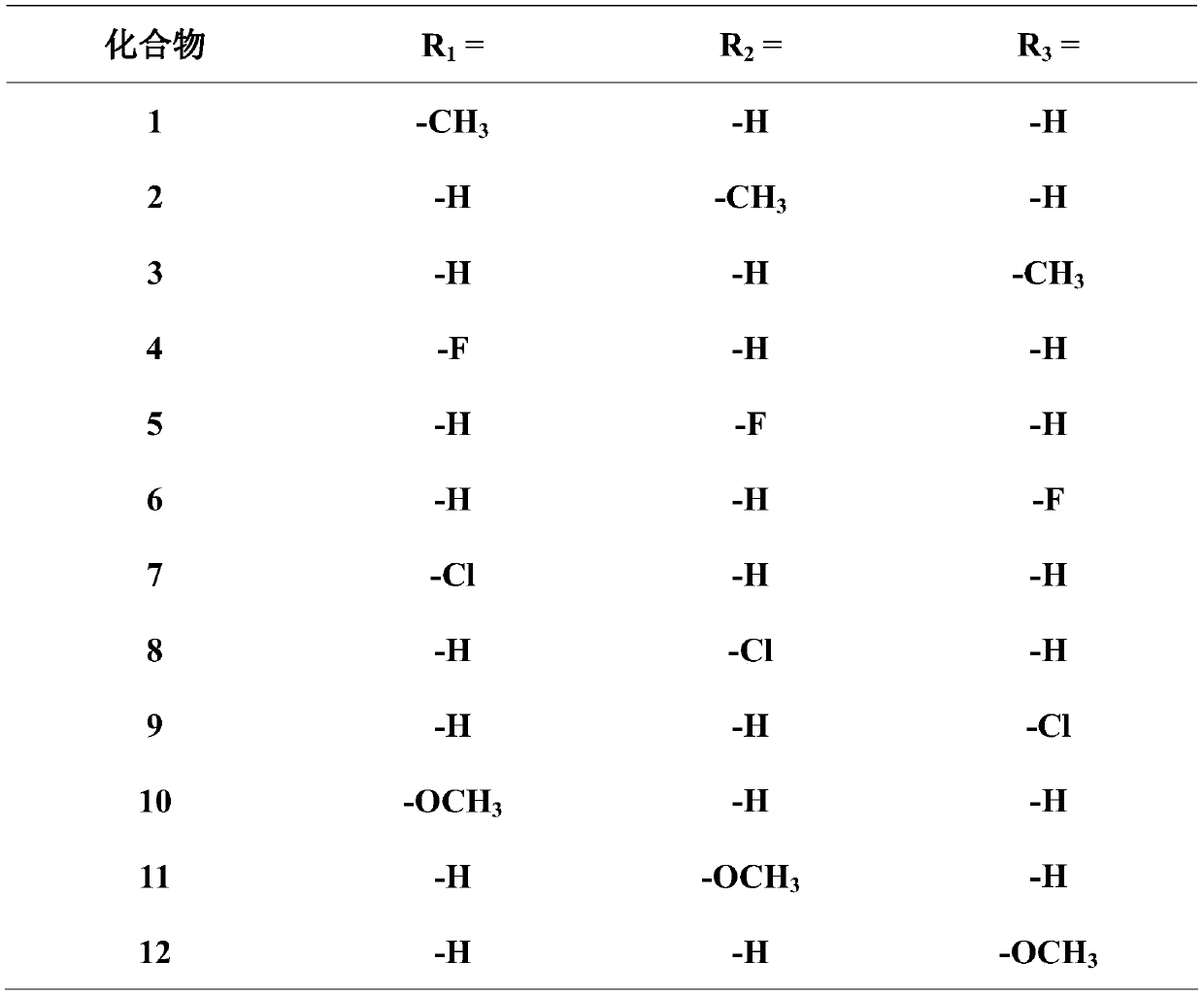
A pleuromutilin derivative with 2-aminoethanethiol side chain and its preparation method and application
A technology of pleuromutilin and aminomethanol, which is applied in the preparation of organic compounds, medical preparations containing active ingredients, preparation of sulfides, etc., can solve the problem of rare pleuromutilin-resistant bacteria and other issues to achieve good antibacterial activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: 22-O-[2-(2-methylbenzamido) ethyl] thioacetyl muulin (compound 1) synthesis
[0057] Intermediate II 0.82g (1.88mmol) was dissolved in 35ml of ethyl acetate, 2-methylbenzoic acid (2.07mmol) and oxalyl chloride 2.07mmol were added, heated and stirred at 70°C for 1 hour to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is stationary phase, sherwood oil: ethyl acetate=1:1 is mobile phase), obtains product 22-O-[2-(2-methylbenzamido) ethyl] thioacetylmyulin ( Pure product of compound 1). Yield 86.21%. HR-MS (ESI): CalforC 32 h 45 NO 5 S(M-H+): 556.3091; Found: 556.3110.
Embodiment 2
[0058] Example 2: Synthesis of 22-O-[2-(3-methylbenzamido) ethyl] thioacetyl muulin (compound 2)
[0059] Intermediate II 0.82g (1.88mmol) was dissolved in 35ml of dichloromethane, 3-methylbenzoic acid (2.07mmol) and tert-butyl chloroformate 2.07mmol were added, heated and stirred at 70°C for 1 hour to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is stationary phase, petroleum ether: ethyl acetate=1:1 is mobile phase), obtains product 22-O-[2-(3-methylbenzamido) ethyl] thioacetylmyulin ( The pure product of compound 2). Yield 83.45%. HR-MS (ESI): CalforC 32 h 45 NO 5 S(M-H+): 556.3091; Found: 556.3107.
Embodiment 3
[0060] Example 3: Synthesis of 22-O-[2-(4-methylbenzamido) ethyl] thioacetyl muulin (compound 3)
[0061] Intermediate II 0.82g (1.88mmol) was dissolved in 35ml of dichloromethane, 4-methylbenzoic acid (2.07mmol) and thionyl chloride 2.07mmol were added, heated and stirred at 70°C for 1 hour to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is stationary phase, and petroleum ether: ethyl acetate=1:1 is mobile phase), obtains product 22-O-[2-(4-methylbenzamido) ethyl] thioacetylmyulin ( The pure product of compound 3). Yield 36.45%. HR-MS (ESI): CalforC 32 h 45 NO 5 S(M-H+): 556.3091; Found: 556.3118.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com