Method for preparing molecularly imprinted polymer used for detecting valnemulin
A technology of molecular imprinting and warnimulin, which is applied in chemical instruments and methods, and other chemical processes, can solve the problems of template leakage and difficulty in obtaining molecularly imprinted polymers, and achieve good hardness, high affinity and selectivity , the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Molecularly imprinted polymers for detection of warnemulin
[0041] The synthesis and preparation steps of the molecularly imprinted polymer in this example are as follows:
[0042] (1) Preparation of prepolymer
[0043] Weigh 0.464g (1mmol) of the vernimulin precursor mock template into a test tube, add 6.0ml of acetonitrile, after ultrasonic dissolution, add 0.342mL (8mmol) of functional monomer methacrylic acid, ultrasonic for 5min, and stand overnight at 4°C. A prepolymer is formed.
[0044](2) thermal polymerization
[0045] Add 4.0mL (20mmol) of ethylene glycol dimethacrylate and 30mg of azobisisobutyronitrile to the above prepolymer, sonicate for 5min, blow nitrogen for 5min, seal, and polymerize in a water bath at 60°C for 24h;
[0046] After thermal polymerization for 24 hours, a bulk polymer was obtained, which is the molecularly imprinted polymer used for the detection of warnemulin in this example.
[0047] Grind the blocky polymer above, pass ...
Embodiment 2
[0049] Example 2 Determination of the performance of molecularly imprinted polymers used to detect warnemulin
[0050] In this example, the molecularly imprinted polymer (with a particle size of 38.5 μm to 75 μm) prepared in Example 1 was selected to be filled in a 3 mL solid-phase extraction empty cartridge (150 mg / column), and activated with 3 mL methanol and 3 mL water in sequence.
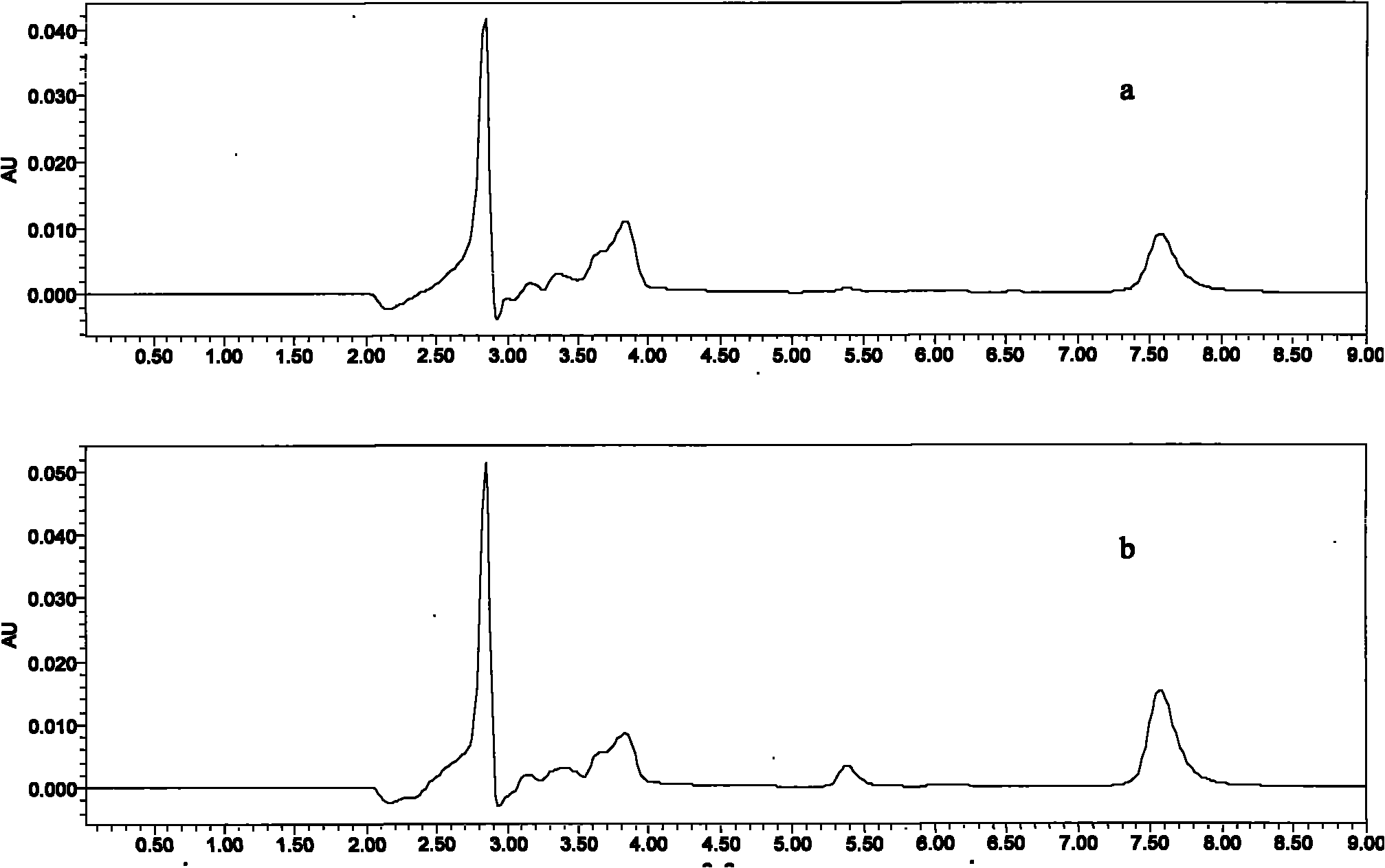
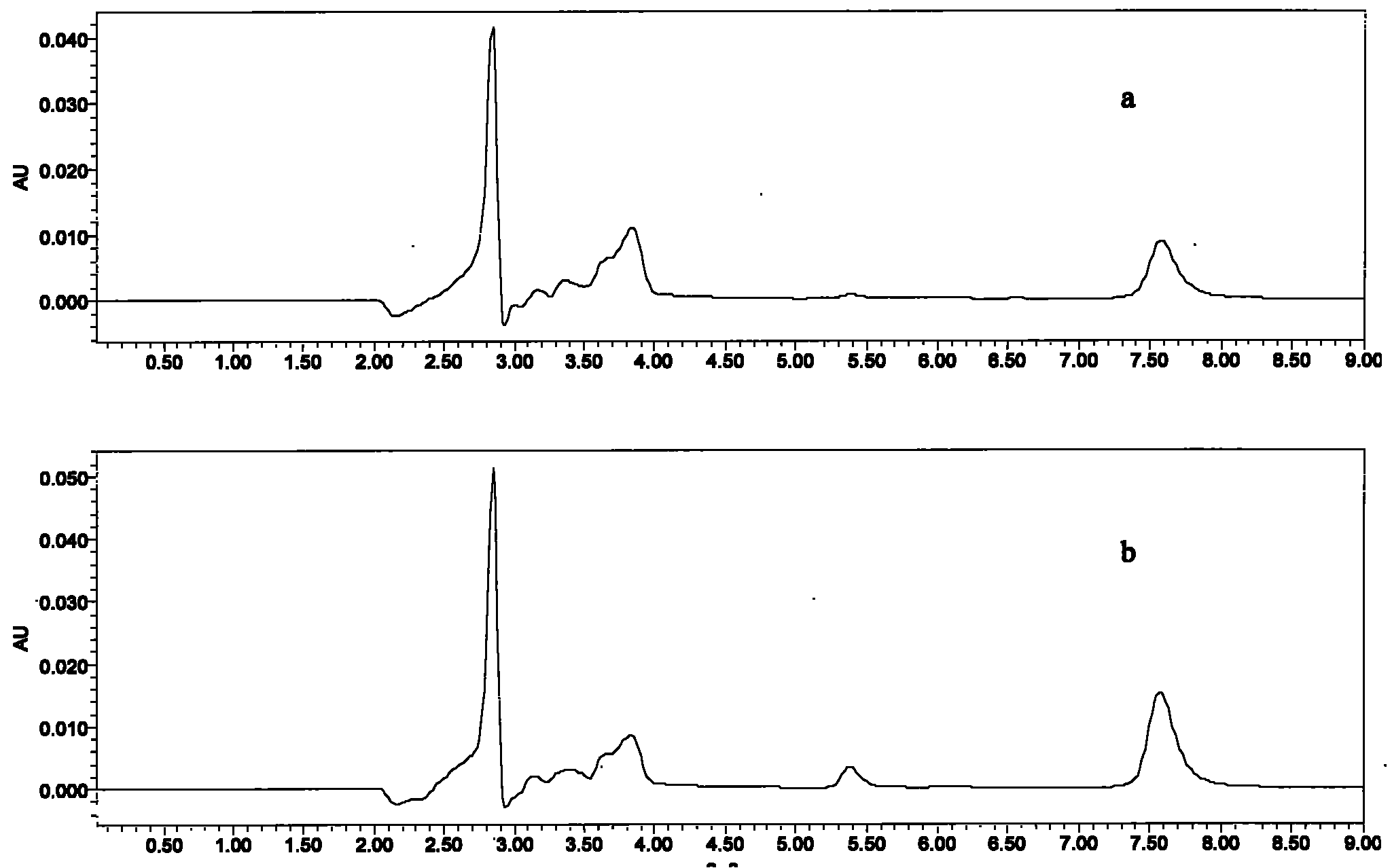
[0051] The samples in this example are 1, 5, 10, and 50 μg / mL series concentration of warnemulin acetonitrile solution, take 2-5 mL of sample, wash with 3 mL of water, 3 mL of acetonitrile, press dry, and use 5% ammoniated methanol 5 mL for elution. Carefully blow dry with nitrogen, dissolve with mobile phase, pass through a needle filter, and detect with HPLC-UV. Test results such as figure 1 As shown, the AU on the ordinate in the figure is the absorbance unit, and the abscissa represents the time in minutes (min). figure 1 Two chromatographic curves are given in , wherein a is the chromat...
Embodiment 3
[0052] Molecularly imprinted polymer solid-phase extraction column prepared in Example 3 is used for the determination of vornimulin in feed
[0053] In this example, the molecularly imprinted polymer (with a particle size of 38.5 μm to 75 μm) prepared in Example 1 was selected to be filled in a 3 mL solid-phase extraction empty cartridge (150 mg / column), and activated with 3 mL methanol and 3 mL water in sequence.
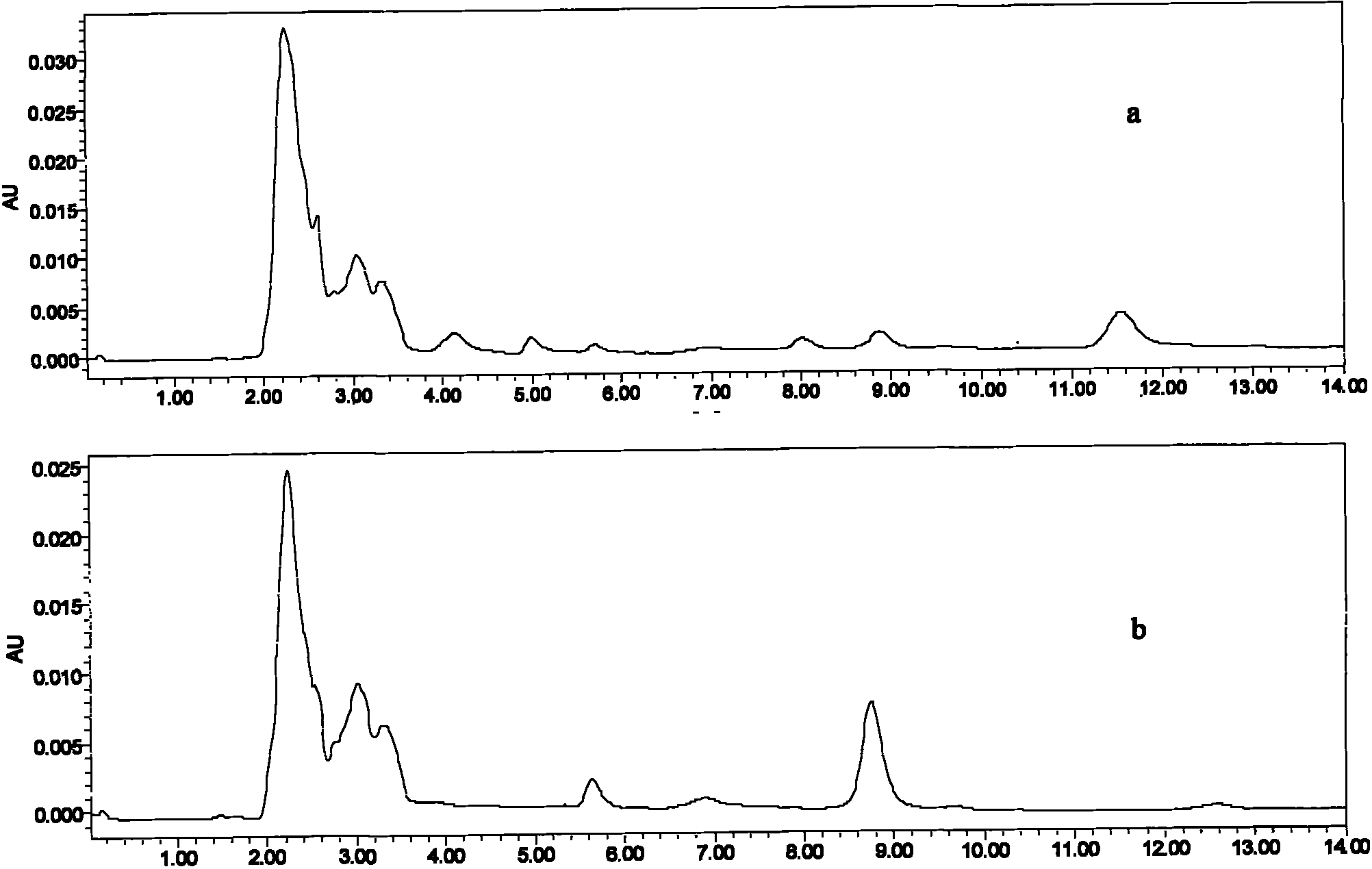
[0054] The samples in this embodiment are extracted from the feed with an organic solvent, blown dry, and dissolved in acetonitrile to prepare 10 and 50 μg / mL acetonitrile solutions of the vernimulin feed matrix. Take 2 mL of sample, wash with 3 mL of water and 3 mL of acetonitrile, dry under pressure, and elute with 5 mL of 5% ammoniated methanol. Carefully blow dry with nitrogen, dissolve with mobile phase, pass through needle filter membrane, and use for HPLC-UV detection. Test results such as figure 2 As shown, the AU on the ordinate in the figure is the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com