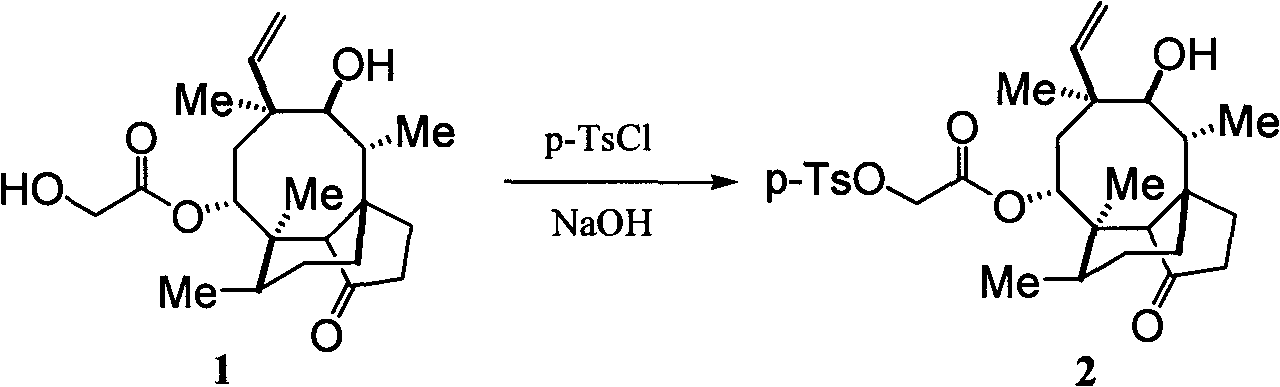Valnemulin synthesis method
A Vonimulin and synthetic method technology, applied in bulk chemical production, sulfide preparation, organic chemistry and other directions, can solve the problems of complex process, low yield and high cost, and achieve simple process operation, high yield, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
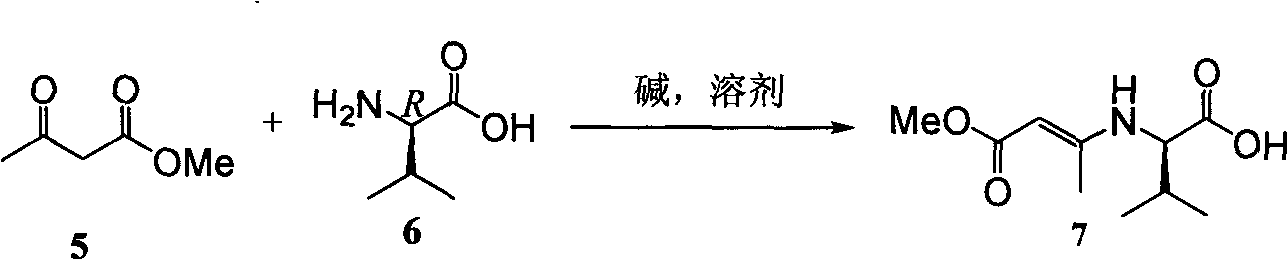
Examples
Embodiment 1
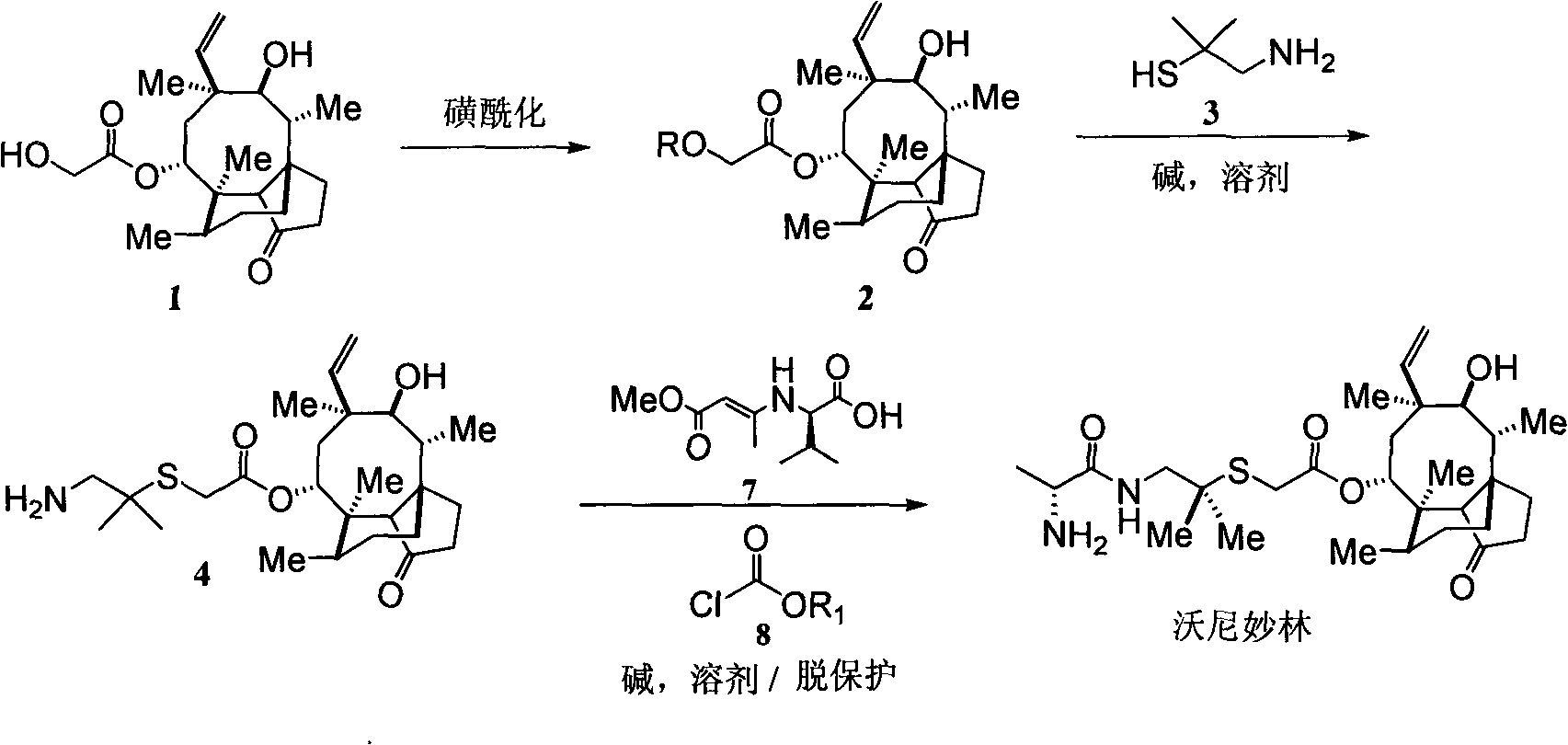
[0031] (1) Synthetic compound pleuromutilin sulfonate (2):
[0032]
[0033] Instruments: 1000mL three-necked flask with reflux condenser, thermometer and addition funnel. Add methyl tert-butyl ether (200mL), water (40mL) into the bottle, and cool to 0°C in an ice-water bath, then add pleuromutilin (1) (75.7g), and stir at 0-3°C for 15 minutes . Add p-toluenesulfonyl chloride (42.0 g), tert-butyl methyl ether (160 mL) and water (120 mL) into the dropping funnel, and slowly drop them into the reaction flask. After the dropwise addition, the resulting suspension was stirred at room temperature for 10 minutes, cooled to 0° C. with an ice-salt bath, and 1N NaOH (50 mL) was slowly added dropwise to the reaction solution. The resulting mixture was heated to reflux for 2 hours. The reactant was cooled to 0°C in ice-brine, and water (400 mL) was added. A large amount of solid formed which was filtered. The solid was filtered out with water, washed with cold ether, and dried to...
Embodiment 2
[0044] (1) Synthetic compound pleuromutilin sulfonate (2):
[0045]
[0046] Instruments: 1000mL three-necked flask with reflux condenser, thermometer and addition funnel. Add methyl tert-butyl ether (200mL), water (40mL) into the bottle, and cool to 0°C in an ice-water bath, then add pleuromutilin (1) (75.7g), and stir at 0-3°C for 15 minutes . Add methanesulfonyl chloride (38.0g), diethyl ether (160mL) and water (120mL) into the dropping funnel, and slowly drop them into the reaction flask. After the dropwise addition, the resulting suspension was stirred at room temperature for 10 minutes, cooled to 0° C. with an ice-salt bath, and 1N KOH (50 mL) was slowly added dropwise to the reaction solution. The resulting mixture was heated to reflux for 2 hours. The reactant was cooled to 0°C in ice-brine, and water (400 mL) was added. A large amount of solid formed which was filtered. The solid was filtered out with water, washed with cold ether, and dried to obtain 46.6 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com