Pleuromutilin derivatives, and preparation method and application thereof
A technology for pleuromutilin and derivatives, which is applied in the field of pleuromutilin derivatives and their synthesis, and can solve the problems of low antibacterial activity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
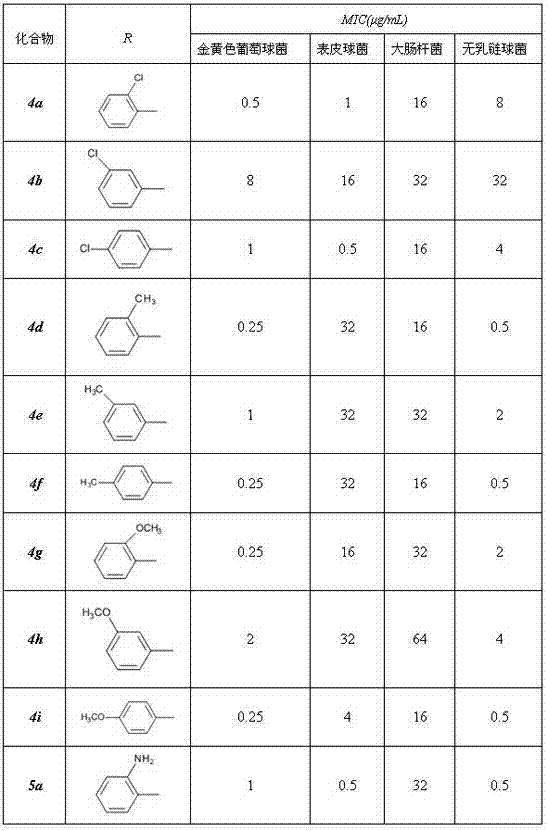
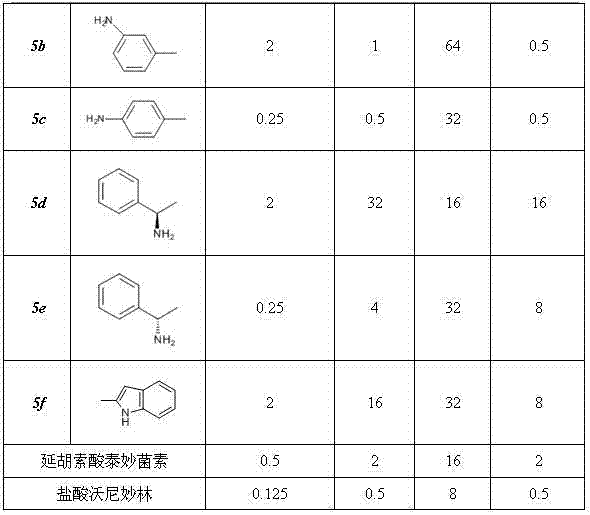
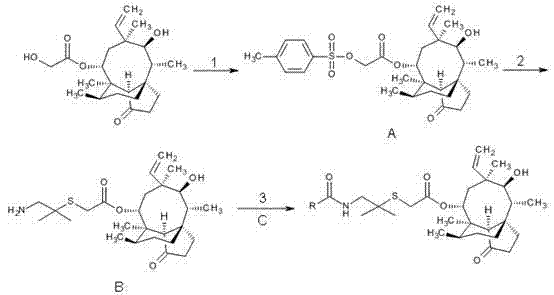
[0042] Example 1. 22-O-(4-toluenesulfonyl)oxyacetylmpirin (A)
[0043] Dissolve 75.7g (0.2mol) of pleuromutilin and 42g (0.22mol) of p-toluenesulfonyl chloride in 200ml of methyl tert-butyl ether, stir the mixture slowly and drop into NaOH solution with a concentration of 0.01mol / ml 50ml. Then heated to reflux while vigorously stirring. After 10-20min, a large amount of white matter was formed, and then stirred for 20-40min. Filter while hot with a Buchner funnel, then rinse with methyl tert-butyl ether (MTBE) and distilled water, and dry naturally to obtain a white powder product (A), with a yield of 97.8%.
[0044] mp 147~148 o C; IR (KBr): 3446, 2924, 2863, 1732, 1633, 1597, 1456, 1371, 1297, 1233, 1117, 1035, 832, 664, 560 cm -1 ; 1 H NMR (400 MHz, CDCl 3) δ 0.63 (d, 3H, J = 6.8 Hz), 0.87 (d, 3H, J = 6.8Hz), 1.11–1.15 (m, 1H), 1.22-1.26 (s, 5H), 1.33–1.36 (m, 1H), 1.41–1.44 (m, 1H), 1.46-1.50 (m, 5H), 1.63-1.65 (dd, 2H,J 1 =10Hz,J 2 =7.2 Hz), 2.01–2.08(m, 3H), 2.21...
Embodiment 2
[0045] Example 2. 14-O-[(1-Amino-2-methylpropan-2-yl)thioacetyl]Multiline (B)
[0046] Cut 0.63g of sodium metal into small pieces and add it to 150ml of absolute ethanol, filter out impurities after the reaction to obtain a sodium ethylate ethanol solution with a mass concentration of 1 to 2%, then add 1.89g (13.5mmol) of Dimethylcysteamine hydrochloride, stirred at room temperature for about 1h, cooled to -5°C, added 7.2g (13.5mmol) of 22-O-(4-toluenesulfonyl)oxyacetylmtilin, and Stir in the bath for 2.5h, distill most of the ethanol under reduced pressure after the reaction, add ethyl acetate to extract, and wash the unreacted dimethylcysteamine hydrochloride and the p-toluenesulfonate generated with distilled water, to the separated organic The phase was dried overnight by adding anhydrous magnesium sulfate, and then separated on a silica gel column (ethyl acetate:ethanol=10:1) or recrystallized (ethyl acetate) to obtain 3.7 g of the target compound (B), with a yield of 59...
Embodiment 3
[0048] Example 3. 14-O-[(2-chloro-benzoyl-2-methylpropan-2-yl) mercaptoacetyl] Mutilin ( 4a )
[0049] Method 1: Dissolve 0.78g (5mmol) of o-chlorobenzoic acid in 30ml of SOCl 2 , heated to reflux for about 4h. Then the unreacted SOCl is reclaimed under reduced pressure 2 , the resulting oil was cooled to room temperature, and 30ml of dichloromethane (DCM) was added for use.
[0050] 1.6g (3.5mmol) of 14-O-[(1-amino-2-methylpropan-2-yl) mercaptoyl] Mutilin and 2.5g of triethylamine were dissolved in 60ml of DCM, in Add the oil obtained from the above reaction dropwise under stirring at around 0°C, and react for another 3 hours after the dropwise addition. After the reaction, the mixture was washed twice with water and washed with saturated NH 4 Cl solution was washed, and after separation, the organic phase was washed with anhydrous MgSO 4 After drying overnight, the DCM was evaporated under reduced pressure, and the resulting mixture was separated by silica gel column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com