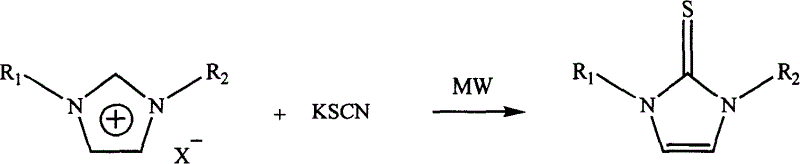Process for microwave synthesis of 1,3-disubstituted imidazole-2-thioketone
A disubstituted imidazole and microwave synthesis technology, applied in 1 field, can solve the problems of methanol poisoning, long reaction time, etc., and achieve the effects of reducing side reactions, shortening reaction time, and reducing microwave radiation power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
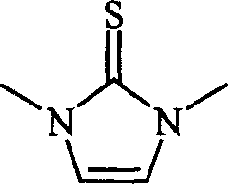
[0014] Example 1 Synthesis of 1,3 dimethylimidazole-2-thione
[0015] Mix 10 mmoles of 1,3 dimethylimidazole chloride and 11 mmoles of potassium thiocyanate uniformly in a 20 ml one-mouth bottle, react under 50W power microwave radiation for 5 minutes, cool naturally to room temperature, and use the reaction mixture with After 50 ml of ethyl acetate-water (volume ratio 1:1) was dissolved, put it into a separatory funnel, separate the organic phase, dry it with anhydrous potassium sulfate for 2 hours, concentrate, and use silica gel column chromatography (eluent (n-hexane: ethyl acetate = 2:1) to obtain 1.02 g of 1,3-dimethylimidazole-2-thione with a yield of 80%.
[0016]
[0017] 1 HNMR (500MHz, CDCl 3 ): δ=3.60(s, 6H), 6.71(s, 2H);
[0018] 13 CNMR (500MHz, CDCl 3 ): δ=34.56, 117.55, 162.12;
[0019] IR (cm -1 )2957, 1445, 1230, 1045;
[0020] MS([M+H] + ): 128.8;
Embodiment 2
[0021] Example 2 Synthesis of 1-butyl-3-methylimidazole-2-thione
[0022] The reaction steps are the same as in Example 1, except that 1-butyl-3-methylimidazole bromide is used as the raw material, and the post-treatment operation is the same as in Example 1, and the yield of the product obtained is 75%.
[0023]
[0024] 1 HNMR (500MHz, CDCl 3 ): δ=0.96(t, 3H), 1.38(m, 2H), 1.75(m, 2H), 3.61(s, 3H), 4.03(t, 2H), 6.71(dd, 2H);
[0025] 13 CNMR (500MHz, CDCl 3 ): 13.74, 19.82, 31.02, 35.10, 47.83, 116.63, 117.74, 161.72
[0026] IR (cm -1 ) 2958, 2933, 1568, 1462, 1414;
[0027] MS([M+H] + ): 170.8;
Embodiment 3
[0028] Example 3 Synthesis of 1-butyl-3-methylimidazole-2-thione
[0029] The reaction steps are the same as in Example 2, except that the reaction is carried out under 70W power microwave radiation for 2 minutes, and the post-treatment operation is the same as in Example 1, and the yield of the product obtained is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com