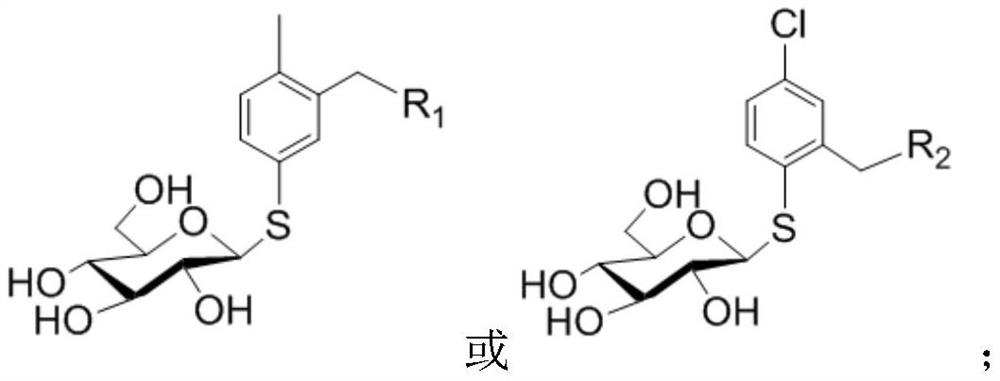
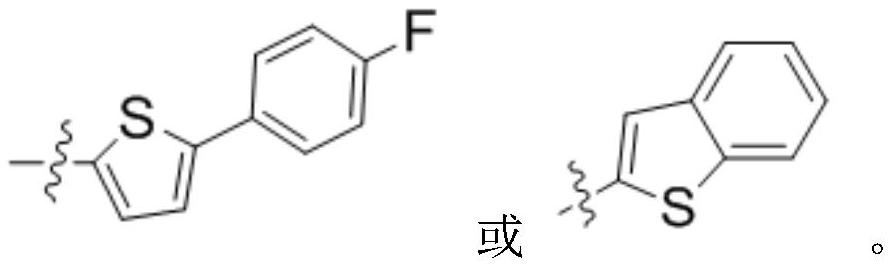
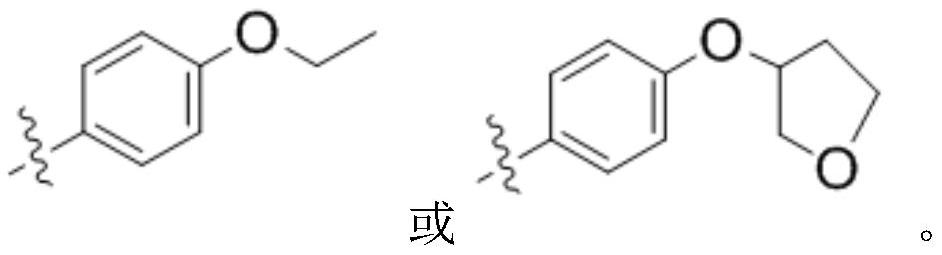
Preparation method and application of peracetyl-protected 1-thioglucose and glucose 1-mercaptan
A technology of glucosinolate and glucose, which is applied in the fields of medicine and sugar chemical synthesis, can solve the problems of inability to proceed, no effect, low solubility, etc., and achieve high reaction efficiency and yield, no irritation and odor, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Weigh 1,2,3,4,6-penta-O-acetyl-D-glucose (1g, 2.6mmol) and dissolve it in ethyl acetate (15mL), add potassium thioacetate (590mg, 5.2mmol), Stir and mix, add boron trifluoride diethyl ether (1.3mL, 10.4mmol), react at a constant temperature of 50°C for 4 hours, cool the reaction solution to room temperature, add triethylamine dropwise to neutralize to pH = 7, concentrate under reduced pressure and column Chromatography yielded 987 mg of 2,3,4,6-tetra-O-acetyl-1-S-acetyl-β-D-glucose with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ): δ5.29-5.24(2H,m,H-3,H-1),5.15-5.08(2H,m,H-2,H-4),4.26(1H,dd,J=12.4Hz,J =4.4Hz, H-6a), 4.09(1H, dd, J=12.4Hz, J=2.0Hz, H-6b), 3.83(1H, ddd, J=10.0Hz, J=4.4Hz, J=2.0Hz ,H-5),2.38(3H,s,SCOCH 3 ),2.07,2.03,2.02,2.00(12H,4×s,COCH 3 ).
Embodiment 2
[0045] Under traditional one-step conditions, direct use of potassium thioacetate leads to extremely low yields:
[0046] Weigh 1,2,3,4,6-penta-O-acetyl-D-glucose (1g, 2.6mmol) and dissolve it in dichloromethane (15mL), add potassium thioacetate (590mg, 5.2mmol), Stir and mix, add boron trifluoride diethyl ether (578 μL, 5.2 mmol), react at room temperature for 24 hours, dropwise add triethylamine to neutralize to pH = 7, concentrate under reduced pressure and column chromatography to obtain 2,3,4,6-tetra -O-acetyl-1-S-acetyl-β-D-glucose 312 mg, yield 30%.
Embodiment 3
[0048] Under the traditional one-step process conditions, the use of potassium thioacetate while using dimethylformamide as a solvent causes the raw materials to not react:
[0049] Weigh 1,2,3,4,6-penta-O-acetyl-D-glucose (1g, 2.6mmol) and dissolve it in dimethylformamide (15mL), add potassium thioacetate (590mg, 5.2mmol ), stirring and mixing, adding boron trifluoride diethyl ether (578 μL, 5.2 mmol), and reacting at a constant temperature of 50° C. for 4 hours, the raw materials did not react, and the yield was 0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com