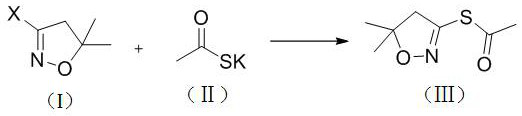S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate as well as synthesis method and application of S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate
A technology of dihydroisoxazole and ethyl ethosulfate, which is applied in the production of organic chemistry and bulk chemicals, can solve the problems of great harm to the environment and human beings, difficult to treat wastewater, etc., and achieves low equipment requirements, fast reaction speed, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] At room temperature, 3-chloro-5-dimethyl-4,5-dihydroxazole (compound) shown in formula (I) was sequentially added in 60 ml of ethanol, and 12.1 g of potassium thioacetate. Then heated to 80 ° C stirring reaction, 4HR was used to detect no 3-chloro-5,5-dimethyl-4,5-dihydro isoxazole, the reaction was completed, and the reaction liquid was completed, and the inorganic salt was removed. After the filtration, the mother liquor was evaporated to evaporase, 17.7.7 g of the oil was 17.7 g, which is S- (5, 5-dimethyl-4,5-dihydrozole-3-yl) ethyl sulfate. . After HPLC detection, the purity of the product was 93%, and the yield was 95.1% in 3-chloro-5,5-dimethyl-4,5-dihydroxazole.
Embodiment 2
[0037]At room temperature, 3-chloro-5,5-dimethyl-4,5-dihydroxazole 13.3 g and potassium thioacetate were sequentially added in 60 ml of acetonitrile, and then heated to 75 ° C stirring reaction, 4HR The non-3-chloro-5,5-dimethyl-4,5-dihydroxazole was detected by HPLC, and the reaction was completed, the reaction solution was cooled to room temperature, and the inorganic salt was removed, and the filtered mother liquor was evaporated. Acetonitrile. After constant weight, 17.9 g of the oil was obtained, which was ethyl ester of S- (5,5-dimethyl-4,5-dihydrooxazole-3-yl). After HPLC detection, the purity of the product was 94%, and the yield was 97.3% in 3-chloro-5,5-dimethyl-4,5-dihydroxazole.
Embodiment 3
[0039] At room temperature, 3-chloro-5,5-dimethyl-4,5-dihydrozole 13.3 g and 12.7 g of thiococciocetate were sequentially added in 60 ml of methanol, and then heated to 65 ° C stirring reaction, 5HR No 3-chloro-5,5-dimethyl-4,5-dihydroxazole was detected with HPLC, and the reaction was completed, the reaction solution was cooled to room temperature, and the inorganic salt was removed, and the filtered mother liquor was evaporated. After constant weight, 17.2 g of the oil were obtained, which was S- (5,5-dimethyl-4,5-dihydrozole-3-yl) ethyl sulfate. After HPLC detection, the purity of the product was 94%, and the yield was 93.5% in 3-chloro-5,5-dimethyl-4,5-dihydroxazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com