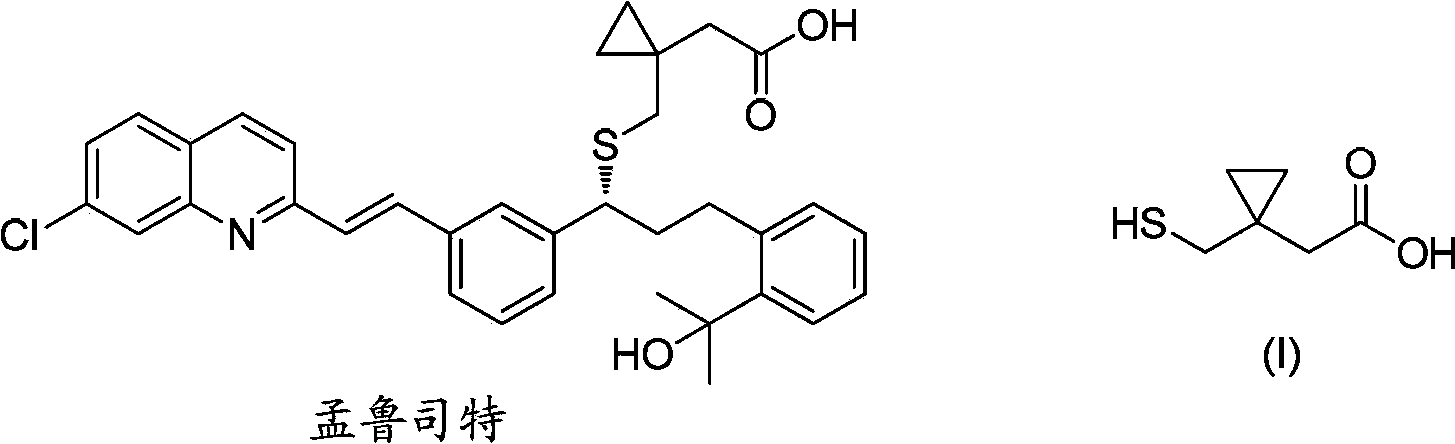
Preparation methods of 1-(mercaptomethyl)cyclopropyl acetic acid and intermediate thereof
A technology of mercaptomethylcyclopropylacetic acid and cyclopropyldimethanol cyclosulfite, applied in the field of preparation of 1-mercaptomethylcyclopropylacetic acid and its intermediates, can solve the problem of poor selectivity and difficult operation and other problems, to achieve the effect of simple and easy process flow, conducive to industrial production, and ingenious design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
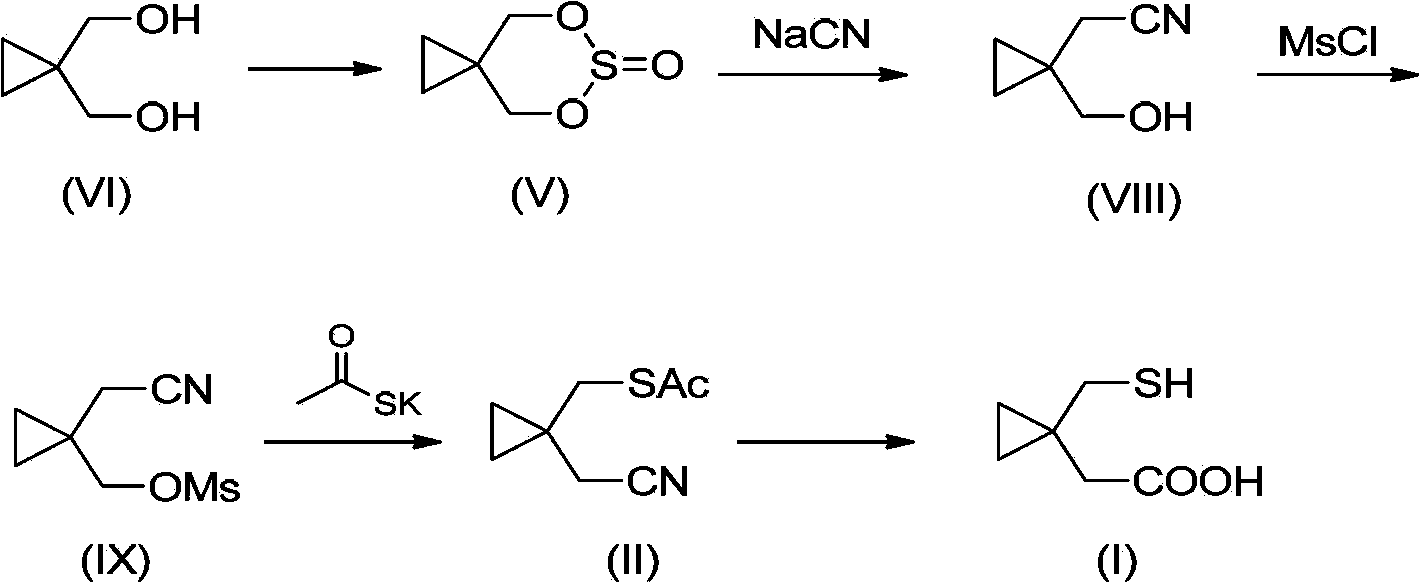
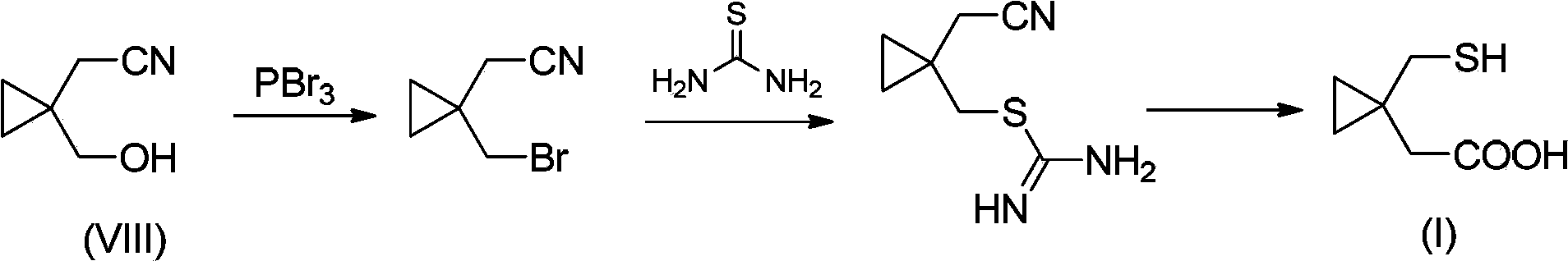
[0029] The general synthetic route of the method for preparing 1-mercaptomethylcyclopropylacetic acid (I) of the present invention is as follows:
[0030]
[0031] Using dibromoneopentyl glycol (VII) as the starting material, cyclization in the presence of zinc powder to obtain cyclopropyldimethanol (VI), and then react with thionyl chloride to obtain cyclopropyldimethanol cyclosulfite (V). Cyclopropyl dimethanol cyclic sulfite (V) is ring-opened with potassium thioacetate to obtain compound (IV), then reacted with methanesulfonyl chloride or p-toluenesulfonyl chloride to obtain compound (III), and then substituted with cyano to form compound ( II), and finally hydrolyze under alkaline conditions to obtain 1-mercaptomethylcyclopropylacetic acid (I).
Embodiment 1
[0033] Embodiment 1: the preparation of cyclopropyl dimethanol (1-2)
[0034]
[0035] Dissolve 25 g of the raw material dibromoneopentyl glycol (1-1) (Suzhou Jinghua Chemical) in 150 mL of ethanol, and add 10 g of zinc powder. Heated to 100°C under nitrogen protection, and refluxed for 4 hours until TLC (PE:EA=1:1 potassium permanganate color development) showed that the raw material point disappeared. Suction filtration, after removing the zinc powder, feed ammonia gas at about 10°C, a large amount of white solids are precipitated, continue feeding ammonia gas until ammonia overflows from the gas outlet. Keep stirring for 30 minutes, filter with suction, wash with a small amount of ethanol, concentrate the filtrate to dryness to obtain a milky white oil, and distill under reduced pressure with an oil pump to obtain 8.3 g of cyclopropyldimethanol (1-2) as a colorless liquid. GC98%, molar yield 85%. 1 H NMR (CDCl 3 ,500Hz)δ4.02(s,2H),3.56(s,4H),0.48(s,4H); MS(ESI)m / z=103...
Embodiment 2
[0036] Embodiment 2: the preparation of cyclopropyl dimethanol (1-2)
[0037]
[0038] Dissolve 25 g of the raw material dibromoneopentyl glycol (1-1) (Suzhou Jinghua Chemical) in 150 mL of methanol, and add 10 g of zinc powder. Heated to 80° C. under nitrogen protection, and refluxed for 6 hours until TLC (PE:EA=1:1 potassium permanganate color development) showed that the raw material point disappeared. Suction filtration, after removing the zinc powder, feed ammonia gas at about 10°C, a large amount of white solids are precipitated, continue feeding ammonia gas until ammonia overflows from the gas outlet. Keep stirring for 30 minutes, filter with suction, wash with a small amount of ethanol, concentrate the filtrate to dryness to obtain a milky white oil, and distill under reduced pressure with an oil pump to obtain 7.3 g of cyclopropyldimethanol (1-2) as a colorless liquid. GC99%, molar yield 75%. See Example 1 for spectrogram data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com