Xylose compound with terminal group containing HS-(PEG)-2-O branch chain and synthesis method of xylose compound
A synthetic method and compound technology, applied in the direction of esterified saccharides, chemical instruments and methods, sugar derivatives, etc., to achieve the effects of mild conditions, reduced consumption of reagents and energy, and simple post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
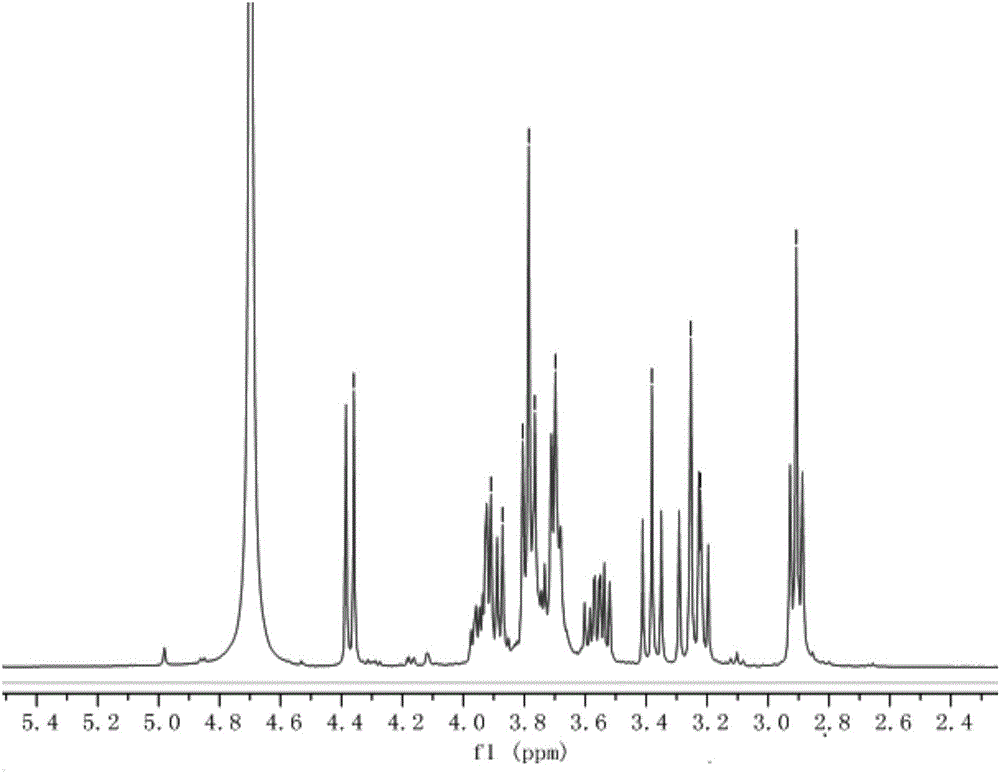
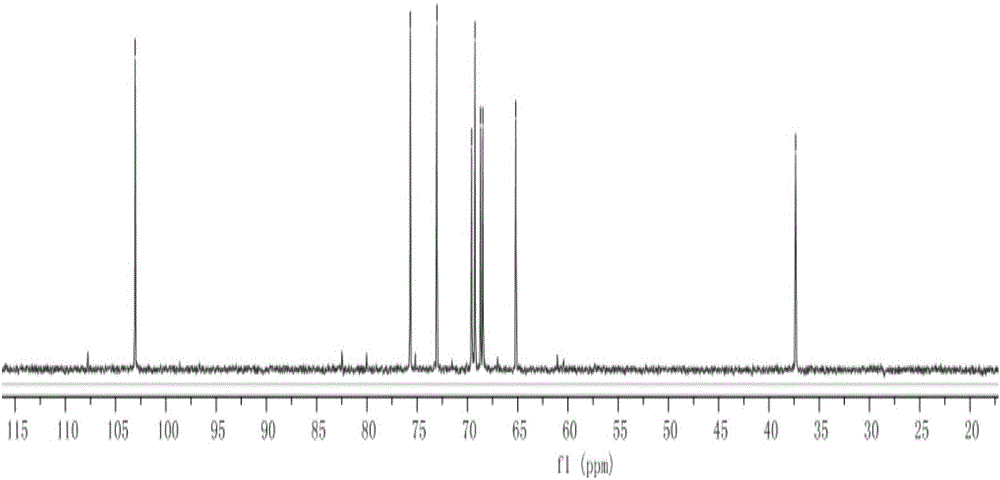
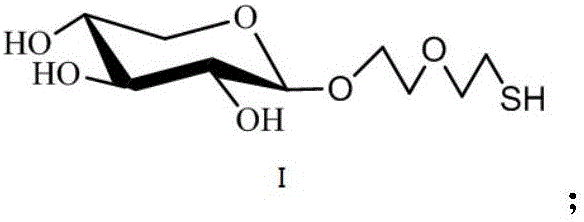
[0021] The end group contains HS-(PEG)2 -O-branched xylose compound, the compound is shown in formula I
[0022]
[0023] The end group contains HS-(PEG) 2 The synthetic method of -O-branched xylose compound comprises the following steps:
[0024] Add 0.4g of iodine and 5g of xylose to the 80mL of acetic anhydride after the redistillation process in a water bath at room temperature, and then react with it. As the acetylation reaction proceeds, a large amount of heat will be released, causing the temperature in the system to rise. When the temperature drops to the greenhouse, add 5g of xylose for the second time. When the temperature drops to room temperature again, add 5g of xylose for the third time until the solution is purple and clear. After the reaction is complete by TLC, dilute the reaction solution with 250mL of dichloromethane , then use 250mL saturated sodium thiosulfate solution to remove excess iodine in the system, then wash with 250mL×4 saturated sodium bicar...
Embodiment 2
[0029] The end group contains HS-(PEG) 2 -O-branched xylose compound, the compound is shown in formula I
[0030]
[0031] The end group contains HS-(PEG) 2 The synthetic method of -O-branched xylose compound comprises the following steps:
[0032] Add 0.3g of iodine and 5g of xylose to the 70mL of acetic anhydride after the redistillation process in a water bath at room temperature, and then add 5g of xylose to react. As the acetylation reaction proceeds, a large amount of heat will be released, causing the temperature in the system to rise. When the temperature drops to the greenhouse, add 5g of xylose for the second time. When the temperature drops to room temperature again, add 5g of xylose for the third time until the solution is purple and clear. After the reaction is complete by TLC, dilute the reaction solution with 150mL of dichloromethane , then use 150mL saturated sodium thiosulfate solution to remove excess iodine in the system, then wash with 150mL×4 saturate...
Embodiment 3
[0037] The end group contains HS-(PEG) 2 -O-branched xylose compound, the compound is shown in formula I
[0038]
[0039] The end group contains HS-(PEG) 2 The synthetic method of -O-branched xylose compound comprises the following steps:
[0040] Add 0.3g of iodine and 5g of xylose to the redistilled 75mL acetic anhydride at room temperature in a water bath, and then react with it. As the acetylation reaction proceeds, a large amount of heat will be released, causing the temperature in the system to rise. When the temperature drops to the greenhouse, add 5g of xylose for the second time. When the temperature drops to room temperature again, add 5g of xylose for the third time until the solution is purple and clear. After the reaction is complete by TLC, dilute the reaction solution with 200mL of dichloromethane , then use 200mL saturated sodium thiosulfate solution to remove excess iodine in the system, then wash with 200mL×4 saturated sodium bicarbonate solution and 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com