Intermediate of sugammadex sodium and preparation method thereof
A technology for sodium sugammadex and an intermediate, which is used in the preparation of an intermediate for sodium sugammadex and the field of preparation thereof, and can solve the problems of increased difficulty, poor process reproducibility, and increased post-processing operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Example 1 Synthesis of 6-perdeoxy-6-perbromo-γ-cyclodextrin
[0045] Under ice bath, add dried γ-cyclodextrin (100g, 77mmol), triphenylphosphine (242g, 925mmol) into DMF (1200ml), stir to dissolve, add NBS (164g, 925mmol, DMF 350ml) dropwise ) solution, control the temperature below 10°C, after 30 minutes of dripping, the color of the reaction solution is dark red and viscous, heat up to 90°C under stirring and continue the reaction for 6 hours, stop the reaction, cool the reaction solution to room temperature and add to ice water (6L) , under stirring, add 4mol / L sodium hydroxide solution until the pH of the system is 8-9, a large amount of solids are precipitated, filtered, the filter cake is beaten with methanol (500ml*2), filtered to obtain a light yellow solid, and Blow drying for 6 hours to obtain 125 g of 6-perdeoxy-6-perbromoγ-cyclodextrin with a yield of 90%.
[0046] Purity HPLC: 98.5%; MS (TOF, ESI + ): 1800.7391[M+H] + .
Embodiment 2
[0047] Example 2 Synthesis of 6-perdeoxy-6 perthioethyl ester-γ-cyclodextrin
[0048] Option One:
[0049] At room temperature, add 400ml of N-methylpyrrolidone to the prepared 6-perdeoxy-6-perbromo-γ-cyclodextrin (40g, 22.2mmol), and add potassium thioacetate (41g, 360mmol) into the system in batches, after the addition, replace the system with nitrogen, 4. Heat the reaction system to 50°C, react for 8 hours, stop heating, add 400ml of ethanol to the system, a large amount of solids are precipitated, vacuum filtration under reduced pressure, and the filter cake is reused Wash once with ethanol / water=1:1 (100ml), dry under reduced pressure to obtain 33.5g of off-white solid, yield: 86%.
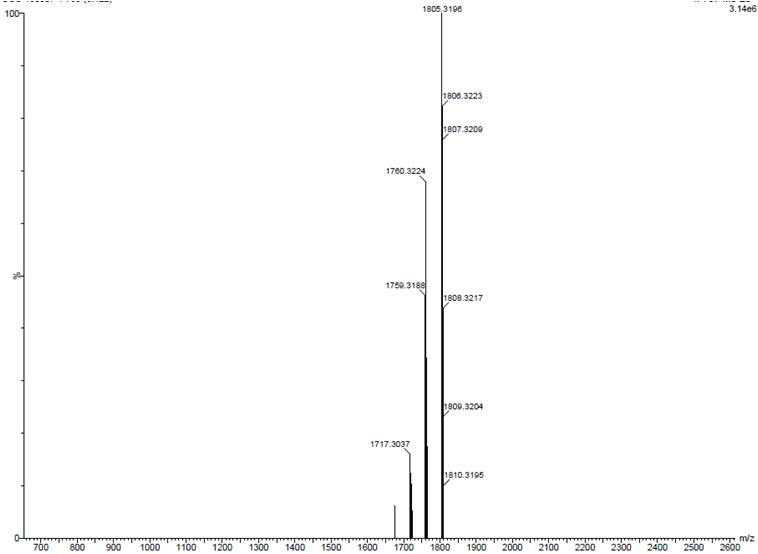
[0050] Purity HPLC: 97%; MS (TOF, ESI - ): 1759.3188 [M-H] - , 1805.3196[M-H+ HCOOH] - .
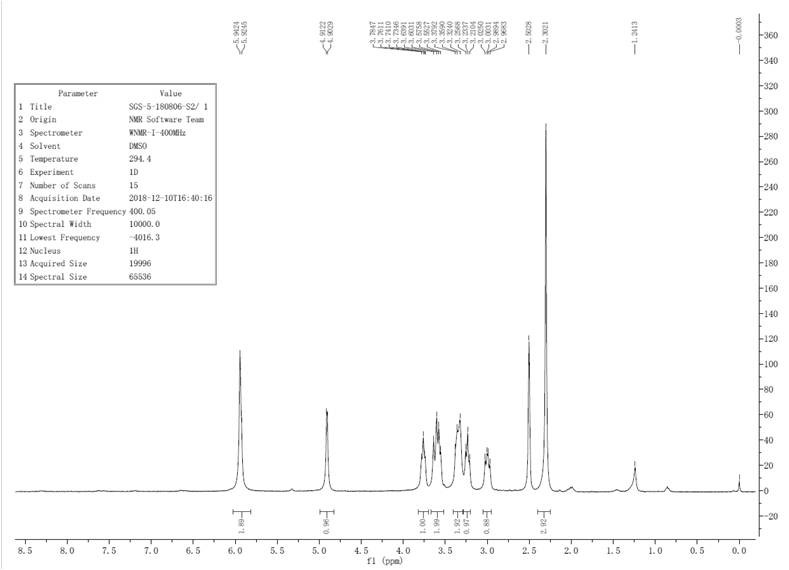
[0051] 1 H NMR (400 MHz, DMSO-d6) δ = 5.94-5.92 (m, 16H), 5.02–4.88 (m, 8H), 3.79 (d, J=10.1, 8H), 3.71–3.55 (m, 8H), 3.43 (m, J=9.8, 8H), 3.26 (m, J=9.3,8H), 2.97 (m, J=13.4, 8H), 2.32 (s, 24H)....
Embodiment 3
[0057] Example 3 Synthesis of Sugammadex Sodium
[0058] Option One:
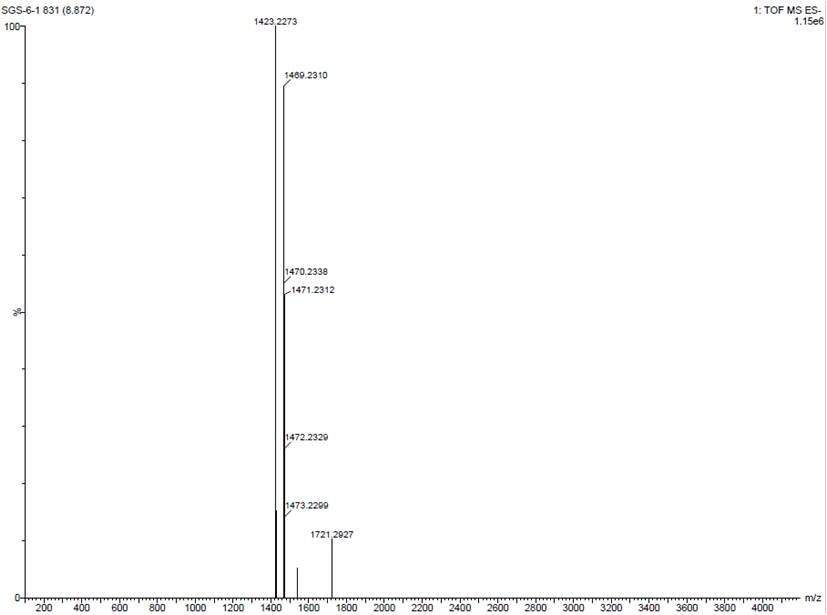
[0059] At room temperature, under nitrogen protection, the prepared 6-perdeoxy-6 perthioethyl ester-γ-cyclodextrin (10g, 5.6mmol) was added to sodium hydroxide solution (100ml, 1.5mol / L), and Complete, after stirring to dissolve completely, continue to stir for 2 hours, and send it to LC-MS (MS (TOF, ESI-): 1423.2273 [M-H]-, 1469.2310 [M-H+ HCOOH]-. Add acrylic acid (8.1g, 112mmol), after 10 minutes of dripping, raise the temperature of the system to 50°C, continue to stir for 6h, lower the system to room temperature, add ethanol (220ml) to the system, a large amount of solids are precipitated, filter the filter cake and beat it with a mixed solvent of ethanol and water , remove residual salts to obtain crude sugammadex sodium, heat and dissolve the crude product with methanol / water, add a small amount of activated carbon for decolorization, and then recrystallize to obtain 9.1 g of refined sugammadex sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com