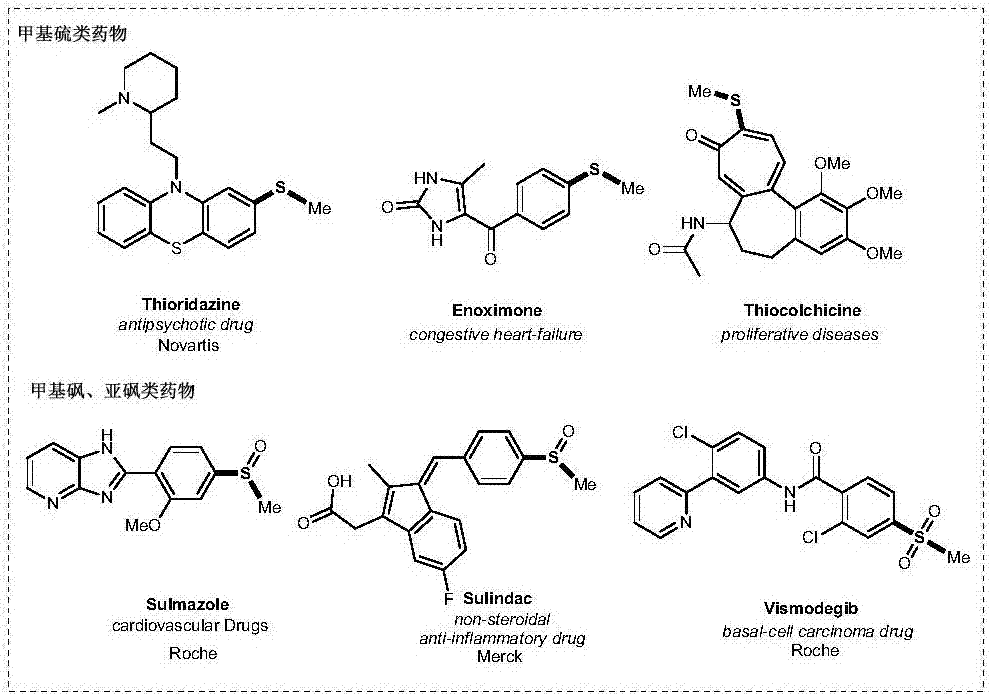
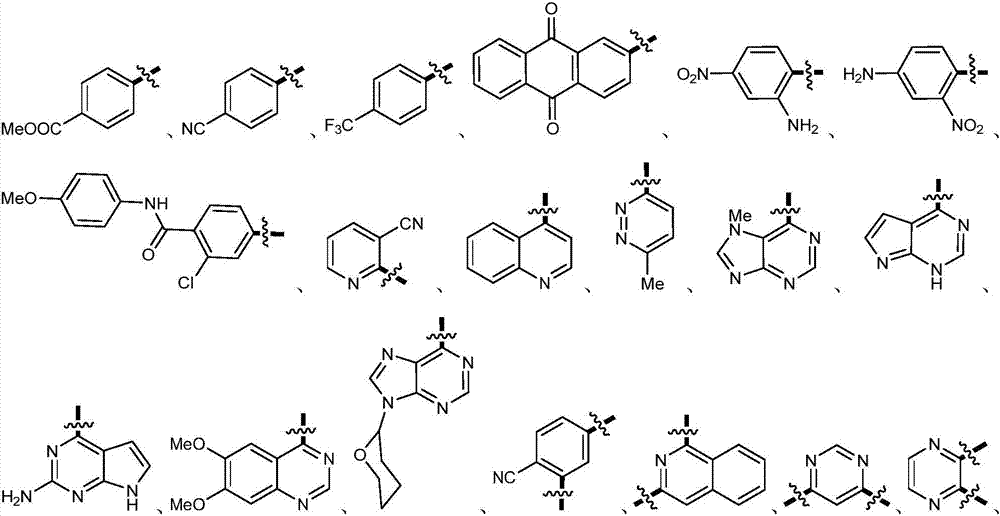
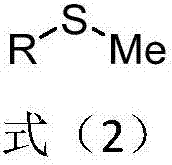Methyl aryl thioether compound, and synthetic method and applications thereof
A technology of methyl aryl sulfide and synthesis method, which is applied in the formation/introduction of mercapto/thioether groups, thioether preparation, drug combination, etc., can solve the problems of high economic cost and low atom utilization rate of methyl iodide, Achieve the effects of high yield, simple reaction operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Under a nitrogen atmosphere, the substrate fenofibrate 1a (0.2mmol, 72.2mg), KSAc (0.6mmol, 68.4mg), DMC (1.0mmol, 90mg), Pd(OAc) was added to a 25mL test tube reactor 2 (0.01mmol, 2.3mg), PPh 3 (0.02mmol, 5.9mg), tBuOK (0.6mmol, 69.2mg), and DMSO (2.0mL). The reaction system was heated to 120°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated ammonium chloride aqueous solution, extracted with ethyl acetate (3*10mL), and purified by column chromatography to obtain 62.5 mg (84%) of product 2a. Rf=0.40 (petroleum ether: ethyl acetate=10:1 ); 1 H NMR (400MHz, CDCl 3 ): δ7.73(d, J=9.2Hz, 2H), 7.70(d, J=8.8Hz, 2H), 7.29(d, J=8.4Hz, 2H), 6.87(d, J=8.8Hz, 2H ),5.14‐5.04(m,1H),2.53(s,3H),1.66(s,6H),1.21(s,3H),1.99(s,3H); 13 C NMR (400MHz, CDCl 3 ): δ194.5, 173.1, 159.4, 144.5, 134.2, 131.7, 130.8, 130.3, 124.9, 117.2, 79.3, 69.2, 25.3, 21.5, 14.9; ,1151,837,761cm ‐1 ; HR...
Embodiment 2
[0047]
[0048] Under a nitrogen atmosphere, the substrate fenofibrate 1a (0.2mmol, 72.2mg), KSAc (0.6mmol, 68.4mg), DMC (1.0mmol, 90mg), Pd(OAc) was added to a 25mL test tube reactor 2 (0.01mmol, 2.3mg), tBuOK (0.6mmol, 69.2mg), and DMSO (2.0mL). The reaction system was heated to 100°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated ammonium chloride aqueous solvent, extracted with ethyl acetate (3*10 mL), and purified by column chromatography to obtain 31.2 mg (42%) of product 2a. The analytical data were the same as in Example 1.
Embodiment 3
[0050]
[0051] Under a nitrogen atmosphere, the substrate fenofibrate 1a (0.2mmol, 72.2mg), KSAc (0.6mmol, 68.4mg), DMC (1.0mmol, 90mg), Pd(OAc) was added to a 25mL test tube reactor 2 (0.01mmol, 2.3mg), tBuONa (0.6mmol, 57.6mg), and DMSO (2.0mL). The reaction system was heated to 100°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated ammonium chloride aqueous solvent, extracted with ethyl acetate (3*10 mL), and purified by column chromatography to obtain 45.4 mg (61%) of product 2a. The analytical data were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com